- Sponsored Content
Principles of Sustainable Development: Establishing a Successful Framework To Drive ChangePrinciples of Sustainable Development: Establishing a Successful Framework To Drive Change
Addressing sustainability can seem insurmountable for any business. Doing so requires endorsement from senior leadership and engagement from an entire organization. To ingrain sustainability into a company’s everyday activities, company leaders must enact strategies that are easy to implement, that produce results quickly, and that will promote long-term goals. Embracing a cycle of continuous improvement is crucial because today’s challenges and innovations can shape the norms of tomorrow, when new challenges inevitably will emerge.
Environmental and social governance lies at the core of our culture at Biogen. Our organization empowers its employees to adopt new ways of working in alignment with an increasing emphasis on sustainability. Biogen’s Product and Technology Development (PTD) team develops and delivers innovative devices, manufacturing processes, product packaging, and analytical solutions. In doing so, we help to provide safe and effective medicines to patients in ways that reduce environmental impacts and promote health equity (Figure 2).
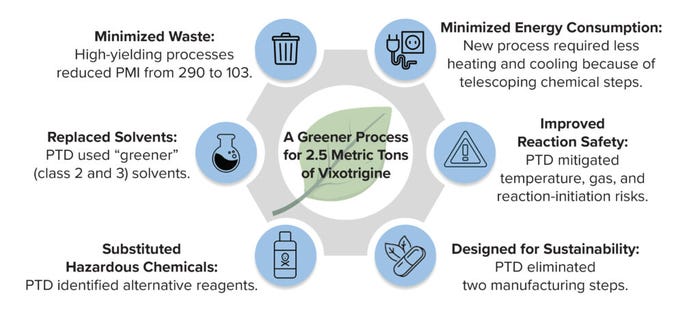
Figure 2: By focusing on key aspects of the drug manufacturing process, scientists behind small-molecule development were able to reduce the total process mass intensity (PMI) of a vixotrigine production process from 290 to 103 kg/kg.

Figure 1A: There are three principles of sustainable product development, the first of which is establishing a baseline.
Envisioning Sustainability
To extend a culture of sustainability throughout the entire organization, Biogen’s PTD sustainability committee developed the principles of sustainable development — a visual tool to drive and track continuous change (Figure 1A–C). The principles were created to facilitate easy implementation, empower and engage employees, and highlight business impacts. Here we highlight three case studies detailing how the principles have promoted sustainability and created safer, more energy-efficient laboratory environments and manufacturing processes for both biologics and small-molecule drugs.
Although our committee’s sustainable-development principles were developed for drug-development organizations specifically, they are applicable to many businesses, and we encourage external adoption of similar practices. We recommend reading this article alongside BPI’s June 2023 article “Creating a Culture of Sustainability,” which provides additional insight into how Biogen developed its principles of sustainable development (1).

Figure 1B: Under the second principle, employees are encouraged to share and showcase green ideas for the benefit of others.ustainable development (1).
The Principles of Sustainable Development
Easy Implementation: Given the rapid pace of everyday business demands, complex change strategies are often unwelcome to employees and can become redundant. The principles of sustainable development were simplified intentionally to ensure access and involvement for all stakeholders and to ease implementation across the organization. Our three defining principles are
• establish baselines
• acquire and share “green” knowledge
• design for safety, sustainability, and health equity.
For each principle, we have developed examples and considerations for application to facilitate meaningful change. Although some examples target product and process developers specifically, many apply to all industries. Broad strategies include
• preventing waste of materials and commodities
• reducing, refining, replacing, renewing, and recycling (the 5Rs) commodities that cannot be eliminated
• choosing clean energy and using it sustainably.
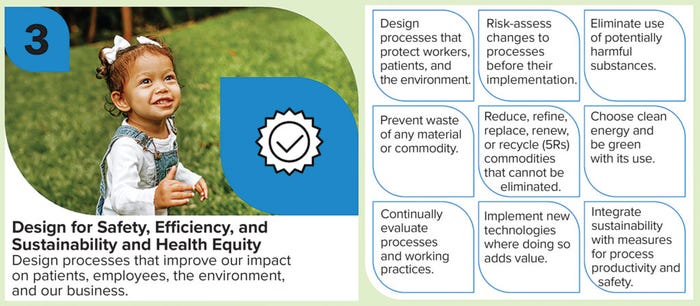
Figure 1C: Finally, a robust sustainability strategy considers the risks and benefits before implementation.
Simplicity drove the design of the principles because straightforward messaging enables continuous improvement and ensures longevity in a changing environment.
Employee Empowerment and Engagement: Business leaders must overcome several hurdles when seeking to enact change. It can be difficult for employees to understand the need for change, to know where to start, and to know how best to focus their efforts. The first principle establishes a baseline to help identify areas of potential improvement. Then, leaders should define measurable ways to assess the potential impact of individual changes.
It is important for employees to gain comfort with implementing changes and exploring new ways to work. To help achieve those goals, company leaders should encourage idea sharing and give their employees avenues for doing so. Such measures can enhance the chance of program success and promote collaborative work. Showcasing improvements to efficiency and safety also can motivate and inspire individuals both within an organization and across the greater community. Those observations form the second principle — to acquire and share green knowledge.
Impact to Business: Company leaders can design, implement, and measure the impacts of changes by understanding established baselines and familiarizing themselves with existing or groundbreaking strategies for enhancing sustainability. Doing so embodies the final principle: designing for safety, sustainability, and health equity. A robust design strategy should consider the risks and benefits of changes before they are applied. In process development, small-scale studies can help clarify benefits before changes are implemented into large manufacturing operations.
Prioritizing sustainability is good for business. By its nature, sustainability promotes a philosophy of prioritizing safety and accomplishing more with fewer resources. Implementing sustainable practices can increase efficiency at work, thereby reducing materials, waste, time, and energy. Removing or reducing harmful chemicals and substances helps to ensure patient safety and protects both employees and the environment. Investing in sustainability initiatives harmonizes with continuous improvement and innovation, both of which are key drivers for successful businesses. Sustainability should be thought of as an extension of existing corporate strategy. It should not be treated as an additional hurdle.
Inspire Other People: Inspiration provides industry professionals with the desire and confidence to act. Our sustainable-development principles are designed to inspire others by showcasing how to achieve easy and effective sustainability improvements. Adoption of sustainability initiatives has a snowball effect. Senior leadership members realize value by investing in such initiatives and employees gain value through participation. Partners, suppliers, and collaborators gain exposure to company values and witness the methods and benefits of sustainability initiatives that they may not have thought possible.
Applying the Principles of Sustainable Development
Implementation is the most important aspect of creating principles of sustainable development. We on the PTD sustainability committee knew that we were on the right track when laboratories in our organization started using our principles as guidance to redefine their operations. The following three case studies showcase different elements of the principles in action and provide examples of ongoing initiatives to raise sustainability in PTD at Biogen.
Case Study 1 — Large-Scale Sustainable Manufacturing Process of Vixotrigine: Vixotrigine is a nonopioid, voltage- and use-dependent sodium-channel blocker undergoing study for managing neuropathic pain. In anticipation of clinical success, projected high demand of the product warranted examining its manufacturing process for improved sustainability. The Biogen Chemical Process Development team redesigned, optimized, and demonstrated a late-stage manufacturing process for vixotrigine to meet the guiding principle of designing for safety, sustainability, efficiency, and health equity. The process was designed to protect workers and the environment, eliminate use of ecologically harmful substances, and integrate sustainability with productivity to ensure maximum business impact. The optimized, environmentally conscious manufacturing process generated 2.5 tons of vixotrigine drug substance. That process was improved in a number of key areas to enhance safety and minimize waste and energy consumption.
Energy consumption was reduced by eliminating cryogenic conditions using flow technology, tailoring the synthetic approach to enable telescoping reactions for the last three steps and improving active pharmaceutical ingredient (API) particle properties to eliminate the need for reblending and roller-compaction steps during drug-product manufacturing. Waste was minimized by redesigning the synthesis route to allow for telescoping reactions, thus reducing isolations of in-process material. The overall route was optimized for high yield, averaging 90% for good manufacturing practice (GMP) steps, with high volumetric efficiency. The process mass intensity (PMI), which measures the total mass used during a given process, was reduced from 290 kg per kg of product to 103 kg/kg (Tables 1 and 2).
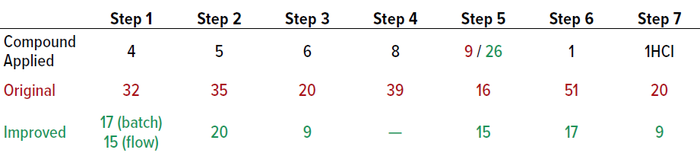
Table 1: Changes in process mass intensity (PMI, kg/kg) of different steps in vixotrigine production.
Case Study 2 — My Green Lab Certification and Freezer Challenge: Biogen started a pilot run for its My Green Lab initiative in 2021 as part of the company’s greater sustainability efforts. My Green Lab (https://www.mygreenlab.org) is a nonprofit organization dedicated to promoting sustainability within academic and industrial laboratories. We conceived of collaboration as an opportunity to exercise our green principles. Our laboratories would establish a baseline by defining their existing green practices with an initial evaluation, then work on acquiring and sharing green knowledge for several months. Improvements would be assessed during a final certification evaluation. However, the enduring value of certification comes when leaders leverage the lessons learned to continue designing laboratories for safety, sustainability, health equity, and efficiency. Such actions follow the program’s guidelines for continuous improvement, which is further assured by the requirement that laboratories recertify every few years.
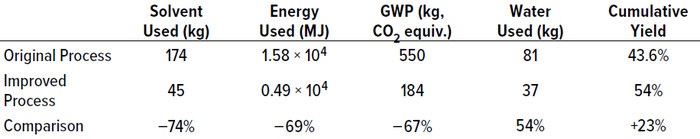
Table 2: Sustainability measures per kilogram of active pharmaceutical ingredient (API)
output (2); GWP = global warming potential.
PTD enrolled nine laboratories in the pilot program, engaging more than 80 employees. Enrolled laboratories experienced significant cultural changes, and each scored within the top-three certification levels. Because of the pilot’s success, PTD enrolled its remaining 18 laboratories in 2022. In total, 27 laboratories were certified, all scoring within the top three levels. The laboratories generated and enacted many sustainability solutions during that process, innovating simple changes such as new approaches to equipment management and complex solutions such as revamping operations to reuse materials that were previously designated for single use.
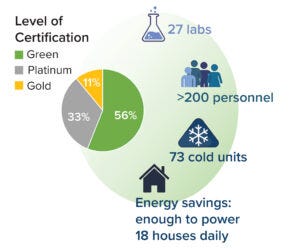
Figure 3: Multiple laboratories in Biogen’s Product and Technology Development (PTD) group implemented sustainability practices for certification in the My Green Lab program. Ultimately, 27 laboratories were certified, with 56% earning the “green” (highest) certification level.
In that same pilot-to-full-scale spirit, the Biologics unit of PTD enrolled 10 laboratories in the 2022 International Freezer Challenge. My Green Lab offers this program to help companies optimize cold storage by defrosting freezers, discarding old reagents, maintaining coils and gaskets, and raising freezer operating temperatures from –80 °C to –70 °C. In all, the 10 PTD laboratories optimized 73 freezer units, which saved 530 kWh of energy per day — enough to power 18 houses. Optimization also saved an estimated US$15,000 per year. Biogen has enrolled all of its 27 laboratories in the 2023 challenge, and we expect to triple the pilot program’s outcome (Figure 3).
Case Study 3 — A Greener, More Efficient, and Higher-Productivity Protein A Chromatography Method: Biogen’s downstream Biologics development team recently undertook efforts to establish a baseline of the sustainability of their platform purification process by evaluating the PMI of each step. The findings indicated that at large-scale manufacturing, the protein A chromatography step contributes about 50% of the platform’s PMI — 1,533 kg/kg out of 3,126 kg/kg, with six other processes sharing the remainder — because large bioreactor volumes are processed over multiple cycles (Figure 4). The impact of the protein A chromatography is further magnified for smaller-scale clinical campaigns because protein A resin is expended to about 20% of its lifetime capacity before being stored or discarded if a clinical program does not continue. It is integral for business leaders to evaluate processes and working practices continuously for sustainable product development. With that in mind, our Process Biochemistry team conducted a technology investment project aimed at providing realistic improvements to the protein A chromatography capture step.
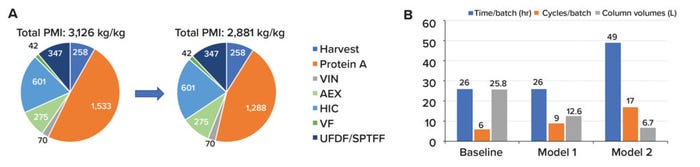
Figure 4: (A) A total process mass intensity (PMI) change of 245 kg/kg in the protein A chromatography step can be achieved with the implementation of reduced process phase volumes and residence times, as investigated in this study. Panel (B) shows modeling of operating scenarios for small-scale manufacturing processes, including the impacts of column size and cycle number on operating time. Assumed conditions included expression titers of 8.5 g/L in a 1,000-L bioreactor, with each campaign comprising four batches. In the models, cutting column size in half led to significant resin savings without affecting the process time.
First, the protein A process was evaluated for potential reductions in both buffer volume and buffer components that could be applied at both large- and small-scale manufacturing. The team targeted the equilibration, chase, and wash phases for improvements. Thorough experimentation culminated in a 16% buffer reduction. Further improvements that could be applied to small-scale manufacturing were achieved by implementing dual–flow-rate loading to increase binding capacity and by reducing nonproduct-contact–phase residence times. The team showed proof of concept with smaller column volumes and rapid cycling, enabling the process to perform more cycles with less resin. It also showed that protein A resin could be used to its full capacity in a single manufacturing campaign of only a few batches. The improved protein A chromatography method provides flexibility for earlier phase programs, reduces total resin costs, and lessens the environmental impact of unused and discarded resins.
The final process changes can be applied to large-scale manufacturing for a 16% buffer reduction per cycle of protein A, about a 16% reduction in the PMI. Additional operating changes applied to small-scale manufacturing could result in a 40% reduction of buffer consumption and 50% reduction of resin volume with no product-quality or process-consistency changes observed. Such changes could save US$260,000–320,000 per manufacturing campaign. Most important, this operating model enables evaluation of time, resin, and buffer constraints that can be modified as needed across programs (Figure 4).
First Steps in the Journey
In a short period, a simple set of sustainability principles has helped drive employee engagement and facilitated efficient and impactful work at Biogen. Improving efficiency and investing in sustainability are both key to business success. We expect our principles to continue accelerating change, and we hope to inspire others to increase sustainability within their own organizations.
Acknowledgments
PTD Sustainability Committee members and collaborators: Armin Delavari, Ashli Polanco, Brad Robertson, Chaomin Li, David Cho, Hossein Hamedi, Kaschif Ahmed, Lanfang Zou, Laurent Dionet, Pamela Whalley, Michael Doane, Adebowale Shoroye, Aeona Magliola.
Green Chemistry, Vixotrigine: Erin O’Brien, Erwin Irdam, Albert Kwok, Dan Patience, Donald Walker, Kenny Tran. My Green Lab Certification and Freezer Challenge: Tooba Gilani, Marshall Bowden. Greener Protein A Chromatography Method: Kashish Malhan.
References
1 Gazaille B, Fahie B. Creating a Culture of Sustainability. BioProcess Int. eBook 21(5)E2 2023: 8–16; https://www.bioprocessintl.com/manufacturing/supply-chains/ebook-working-together-is-key-to-bioprocess-sustainability.
2 Jiménez-González, et al. Expanding the Boundaries: Developing a Streamlined Tool for Eco-Footprinting of Pharmaceuticals. Org. Process Res. Dev. 17(2) 2013: 239–246; https://doi.org/10.1021/op3003079.
Clare Blue is director of biologics development, Allyson Dilks is senior engineer of protein development, Suzie Opalka is associate director of small molecule development, Wenli Liang is senior associate scientist of small molecule development, Brian Fahie, PhD, is vice president and global head of analytical development, and Adriana Rimesso is senior scientist of analytical development at Biogen, 225 Binney Street, Cambridge, MA 02142.
You May Also Like






