- Sponsored Content
- Continuous Bioprocessing
Proud Partners: Advancing Technologies Enable Collaboration and InnovationProud Partners: Advancing Technologies Enable Collaboration and Innovation
August 22, 2022
Sponsored by WuXi Biologics
Recent global events have demonstrated a growing demand for biologics to be made rapidly, cost-effectively, and in high volumes. There has been an urgent need to develop highly flexible and cost-effective next-generation biomanufacturing solutions that provide high yields of therapeutic proteins. Novel technology platforms such as single-use bioreactors and continuous bioprocessing technologies have contributed greatly to improvements in product quality and productivity while reducing cost of goods.
Continuous Bioprocessing Expands
Historically, continuous-culture processes were used for production of low-titer, low-stability, and labile proteins. Now the technology is applied increasingly to other biologics, including more stable modalities such as monoclonal antibodies (MAbs), bispecific antibodies (bsAbs), fusion proteins, and other recombinant proteins. Continuous biomanufacturing approaches have contributed to our ability to meet growing demands for biotherapeutics around the world. Perfusion cell culture has been adopted widely for clinical and commercial manufacturing, offering significant advantages over fed-batch cultures in yield, quality, flexibility, and cost-effectiveness.
A well-developed perfusion culture system such as the WuXi Biologics WuXiUP ultrahigh-productivity platform (Figure 1) can enable 5× to 10× improvement over fed-batch options in cell density and productivity for nearly any type of biologics. Additionally, using continuous product harvesting reduces the residence time of protein products inside bioreactors, leading to improved product quality. We should expect to see more applications of continuous bioprocessing to biomanufacturing in the future. Establishment of such platforms represents an ongoing shift in the industry to address the growing demand for affordable, high-quality, and high-volume biologics.
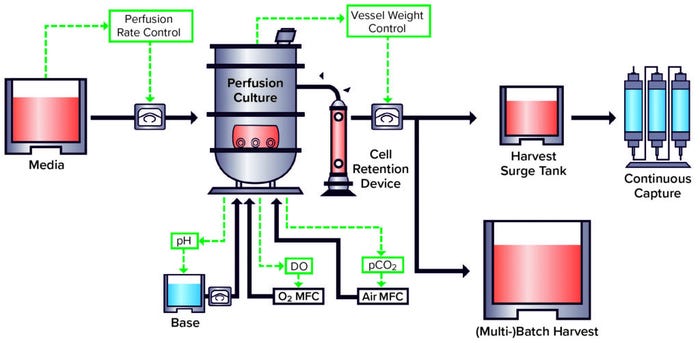
Figure 1: WuXi Biologics WuXiUP ultra-high–productivity continuous biomanufacturing platform
Shortening Timelines
Over the past five years, WuXi Biologics has consistently reduced the standard timeline of chemistry, manufacturing, and controls (CMC) development activities. The time span from initiation of cell-line development to investigational new drug (IND) filing has been shortened from 24 months to 12 months. We accomplished this dramatic improvement by offering a highly vetted and truly one-stop discovery, development, and good manufacturing practice (GMP) manufacturing platform. The knowledge we have gained has allowed us to drive timelines down even further when infectious disease concerns required more expedited development.
In multiple cases, we have reduced the now-standard 12-month timeline down to nine months, and then to six months. We further achieved a record-breaking 2.5-month timeline for a number of anti-COVID-19 neutralizing MAb projects. In addition, timelines from IND to emergency use authorization (EUA) or biologics licensing application (BLA) have been shortened dramatically. Since COVID-19 emerged, our industry has demonstrated its ability to act both quickly and collaboratively. WuXi Biologics has applied integrated and transformative methodologies without compromising product quality or safety to accelerate development timelines for multiple SARS-CoV-2 neutralizing MAbs.
In one significant example, a MAb went from the start of cell-line development activities to EUA within an unprecedented 14 months. Thousands of kilograms of drug substance were manufactured for therapeutic application across the globe. This achievement demonstrates that, in some cases, we no longer need to wait as long as 10 years to develop a new biologic. What we have learned from the COVID-19 projects can be applied to expedite development of treatments for cancers, autoimmune disorders, rare diseases, and more.
From Developer to Enabler
Before WuXi Biologics, I worked with multiple CMC teams in senior leadership positions, including Genzyme (now Sanofi), PDL BioPharma, and Merck. A common characteristic of those companies is that they all develop their own products, with specific teams assigned to the development of each candidate. A common sentiment across professionals in the industry years ago was that no matter which company you work for, you will be involved in the development of only a few new drugs during your career.
Technology platform companies such as WuXi Biologics offer a compelling opportunity to work on and to bring forth many more products for our clients and the market. Through our integrated technology platforms, we have built comprehensive development capabilities and extensive manufacturing capacities that enable hundreds of our global partners to discover, develop, and manufacture biologics of different modalities. It has been extremely rewarding to be involved in delivery of critical new biologics medicines and vaccines into clinical studies and beyond for the benefit of patients worldwide.
During my 10 years at WuXi Biologics, I’ve helped to build a large drug-development organization and led my teams to develop multiple integrated technology platforms. Those include the WuXian custom protein-generation platform, the WuXia cell-line development technology, and the WuXiUP continuous-bioprocessing system. Our WuXiDAR4 novel bioconjugation technology platform ensures consistent drug/antibody ratios (DARs) and antibody–drug conjugate (ADC) homogeneity. All these platforms enable our partners to develop hundreds of biologics more efficiently and cost-effectively. And I have been transformed from a “developer” into an “enabler.”
What also makes me proud is that I’ve been able to inspire many young professionals to grow and advance in their career paths. My teams have enabled more innovative biologics drugs to reach the clinic and market than I ever could have imagined, and witnessing the contributions of those highly trained and knowledgeable young scientists to the global healthcare industry (today and in the years to come) keeps me energized and feeling positive about our future to serve patients better.
Outsourcing Is Here to Stay
In my career, I have seen how the pharmaceutical industry has expanded into biologics and how service companies have evolved accordingly. Before the 2000s, biopharmaceutical industry innovations were driven mostly by the largest pharmaceutical companies through capabilities enabled by vast resources. After 2000, we witnessed the rapid development of biotechnology and similar growth of small companies with strong innovation abilities and technology platforms fueled by venture capital. Many such companies begin with new technologies and/or product candidates transferred from basic research and discovery organizations — or even just concepts discovered through work in academia.
WuXi Biologics recognized that trend and acted to provide the resources and capabilities those small companies required to develop their products successfully for clinical testing and commercial sale. We saw demand increasing from such companies for outsourced development and manufacturing services, especially related to novel modalities such as ADCs and bsAbs. Foreseeing the demand for end-to-end services, we built a comprehensive open-access platform that would enable the scientific teams at start-up companies to focus on their R&D efforts rather than development and manufacturing infrastructure and the required investments. Contract research, development, and manufacturing organizations (CRDMOs) such as WuXi Biologics can improve manufacturing efficiencies and lower the costs of drug development. Ultimately, we can contribute to providing patients with more accessible and affordable biologics in shortened timeframes.
We call our business model a “follow and win the molecule” strategy: working to provide start-ups as well as larger biotechnology and pharmaceutical companies with one-stop services all the way to the clinic and market. Through this strategy, we have enabled more than 500 programs — 156 of them in 2021 alone — for more than 470 clients around the world. Through those hundreds of programs, we have enabled clients to move their programs into clinical trials, and we are proud to say that more than 30 of those products now are in phase 3 and that nine products have been approved for commercial marketing worldwide. Those are the real results of our strategy. Based on our success in enabling so many clients and projects, I believe that biologics service companies such as WuXi Biologics have been fully integrated into the drug-innovation ecosystem and are more important now to the biopharmaceutical industry than ever before.
Dr. Weichang Zhou is chief technology officer and executive vice president of WuXi Biologics, 108 Meiliang Road W, MaShan Binhu District, Wuxi 214092, China; [email protected]; https://www.wuxibiologics.com. WuXia, WuXiUP, and WuXiDAR4 are registered trademarks of WuXi Biologics.
You May Also Like






