- Sponsored Content
- Cell Culture Media
- Process Development
The Critical Role of Media in Intensified Upstream ProcessesThe Critical Role of Media in Intensified Upstream Processes
Sponsored by Millipore Sigma
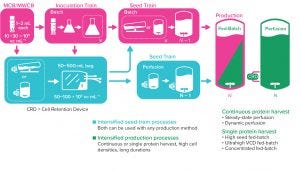
Figure 1: Perfusion can be used to increase viable cell density in the seed train to inoculate the production bioreactor with a higher cell density and in the production bioreactor.
As the need for novel therapeutics increases, so does pressure on the biopharmaceutical industry to improve productivity, accelerate development, increase, and reduce costs — all while ensuring drug product quality. Upstream intensification strategies such as perfusion culture can address those challenges and achieve higher protein titers that can translate into higher throughput, improved flexibility, and compressed timelines.
Successful implementation of perfusion culture or the transition to perfusion from fed-batch culture requires a different and strategic approach to media selection, not just for the bioreactor itself, but for clone selection, cryopreservation, and cell expansion. Here, we explore the foundational role of media across the upstream workflow, particularly for intensified processes, and how high cell density cryopreservation media can deliver important operational benefits.
Just fill out the form below to read the full article now.
Melanie Brandl, PhD, is global product manager, upstream catalog media, and Habib Horry is associate director of marketing for upstream integration and next-generation bioprocessing, both at MilliporeSigma. The life science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the United States and Canada.
You May Also Like






