- Sponsored Content
- Viral Clearance
Using CHO-Endogenous RVLPs for Retroviral Clearance StudiesUsing CHO-Endogenous RVLPs for Retroviral Clearance Studies
Sponsored by Cygnus Technologies
Early evidence of budding C-type particles from Chinese hamster ovary (CHO) cells was documented in the 1970s and 1980s (1, 2). In the early 1990s, scientists from Genentech began to characterize these “retrovirus-like particles” (RVLPs) from CHO cells (3). Although proven to be noninfectious (1, 2), the endogenous particles caused regulators to require a demonstration of retrovirus clearance before clinical trials or market approval authorization (4). To accomplish that, the biopharmaceutical industry adopted the use of xenotropic murine leukemia virus (XMuLV) as a model retrovirus for contract research organization (CRO)–led spiking studies. In the meantime, scientists continued to characterize CHO-endogenous RVLPs, sequencing the proviral genome (5) and using that information to develop highly sensitive detection assays based on real-time quantitative polymerase chain reaction (RT-qPCR) (6). Not long after that, process development scientists began leveraging that knowledge to conduct CHO-endogenous RVLP clearance studies.
The first studies used RVLPs already present in harvested cell culture fluid (HCCF) to determine removal through protein A affinity separation (typically the first process step in a CHO-based monoclonal antibody (MAb) purification process) (7, 8). Those studies succeeded not only in demonstrating the feasibility of the concept, but also in demonstrating the comparability of RVLP to XMuLV clearance (the gold standard for measuring retroviral clearance). Later studies use RVLPs to demonstrate the contributions of further downstream process steps to retroviral clearance, namely anion-exchange (AEX) modes of chromatography (9–11). To accomplish that, and because most endogenous particles had been depleted by the protein A and viral- inactivation steps, RVLPs were spiked back into the process through the addition of concentrated HCCF. Those studies further highlighted the utility of RVLPs in demonstrating retroviral clearance.
In September 2022, global regulatory agencies (including the US Food and Drug Administration, FDA) endorsed a draft revision to the ICH Q5A guideline, which governs the biotechnology industry on the topic of viral safety and evaluation (12). The document stated that “for CHO cell-derived products, CHO-derived endogenous virus particles [RVLPs] can also be used for viral clearance experiments.” Concurrently, Cygnus Technologies developed and launched the MockV RVLP assay to offer a novel approach that enables the use of RVLPs for downstream spiking applications. Packaged as a complete, ready-to-use solution, the kit contains CHO-derived, noninfectious RVLPs that act as biosafety level 1 (BSL-1)–compatible spiking agents for viral clearance testing. Using them enables RVLPs to be handled safely, which overcomes the cost and complexity barriers of conducting live XMuLV studies. This technology allows the incorporation of viral clearance into quality by design, design of experiments, and high throughput screening approaches to process development/characterization — which has remained a challenge.
Prior to product launch, Cygnus Technologies collaborated with several industry partners. They challenged the MockV RVLP kit with a diverse panel of in-process feedstreams and separation techniques to ensure broad accuracy and dependability. Log-reduction value (LRV) results from those studies were compared with historical XMuLV LRV results.
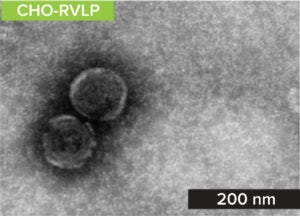
Figure 1a: TEM image of the RVLP stock solution provided in the MockV RVLP kit.
Materials and Methods
RVLPs: Noninfectious RVLPs were produced in CHO cell cultivation. HCCF was purified through multiple modes of chromatography to enrich RVLPs, which then were concentrated to a final stock solution of 1010 particles/mL. Transmission electron microscopy (TEM) was used to visualize particles and determine morphology (a). RVLPs were characterized through dynamic light scattering (DLS) and zeta-potential techniques. Results were compared to those of live XMuLV (supplied by Texcell, NA). The DLS results demonstrate that RVLPs are monodisperse in the stock solution and exhibit an average diameter of 193 nm, whereas XMuLV assays also are monodisperse but exhibit a slightly larger diameter (Figure 1b). Zeta- potential results indicated that the surface charges of each particle were comparable and slightly acidic (Figure 1c).
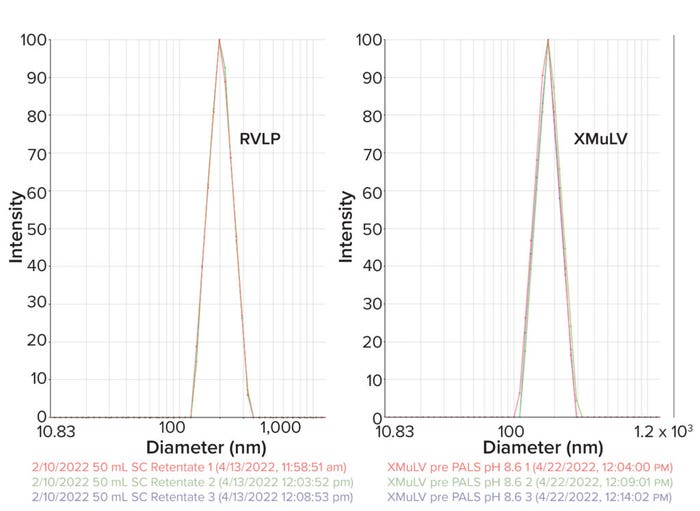
Figure 1b: DLS profiles of both RVLP and XMuLV along with underlying data table showing effective diameter and polydispersity.
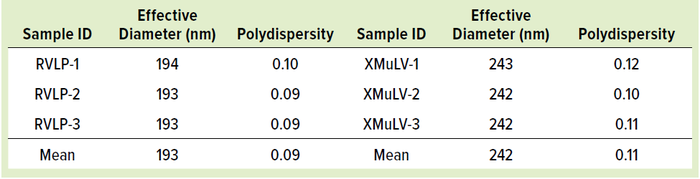
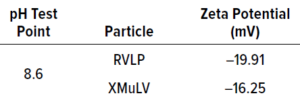
Figure 1c: Test results showing zeta-potential measurement of each particle.
In-Process Materials and Spiking Study Method: MAb-containing material, provided by several collaborators for RVLP spiking studies, included loads from protein-A, AEX, cation-exchange (CEX), and mixed-mode chromatography (MMC) processes as well as samples from viral-filtration steps. For spiking experiments, these load materials were spiked with 1% (v/v) additions of RVLP stock solution and then processed through their respective separation steps. Samples were collected from a number of separation phases (e.g., flowthrough, wash, and elution) and analyzed. LRVs were determined accordingly. In most cases, historical XMuLV LRVs were available for comparison (derived from CRO-led experiments using the same feedstream, separation technique, and operating parameters).
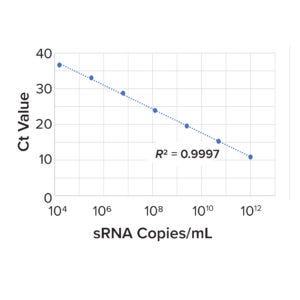
Figure 2: Example sRNA standard curve.
RVLP RT-qPCR Analytical Method: To analyze the concentration of noninfectious RVLPs in samples, the MockV RVLP kit contains RNA extraction and RT-qPCR components. In short, samples are added to a 96-deep-well plate and treated with an endonuclease to degrade proviral RVLP DNA sequences or exogenous RVLP RNA. RNA then is extracted from RVLP and precipitated with a set of proprietary buffers. The plate is stored at –20 °C for 30 minutes, and RNA is pelleted through centrifugation at 3,000g for 20 minutes at 4 °C. After washing and final pelleting, the RNA is resuspended in a proprietary buffer. 10 μL of sample is transferred from each well of the 96-deep-well plate to a qPCR plate containing TaqMan primers/probes directed against the pol region of the CHO-RVLP genome. To determine the quantity of particles in unknown samples, threshold cycle (Ct) values are interpolated into a standard curve generated by a dilution series of sRNA standard provided in the kit (Figure 2). From those concentration values, RVLP LRVs for each experiment can be calculated.
RVLP Spiking Experiment Results
Table 1 summarizes results from RVLP spiking experiments conducted by several industry collaborators. The data demonstrate a good correlation between RVLP- and XMuLV-derived LRV results among all modes of separation tested. In instances when incomplete XMuLV removal was experienced (with protein A and CEX), RVLP LRV values were slightly lower, indicating that RVLPs could be considered as a worst-case model. In instances when complete XMuLV removal was achieved (VF, MMC), complete RVLP removal was achieved as well. For AEX, no comparable XMuLV data were available; however, complete RVLP removal was anticipated during flowthrough centerpoint and bind–elute operations, whereas a low LRV was expected by operating in flowthrough mode with higher conductivity in the load. Those expectations were met when complete clearance was achieved for flowthrough centerpoint and bind–elute, whereas flowthrough at high conductivity resulted in a significant drop in particle reduction (LRV of 1.31).
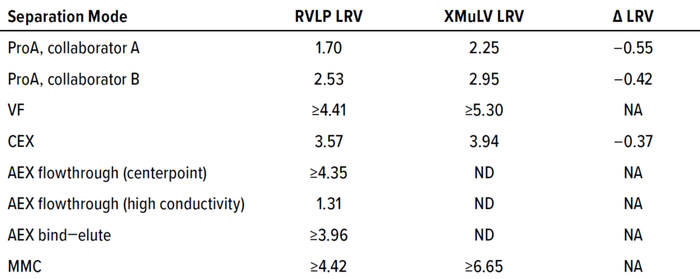
Table 1: Summary of RVLP and XMuLV removal results; LRV = log reduction value; ND = not detected; NA = not available.
The MockV RVLP Kit
Cygnus introduced the MockV RVLP kit (Cat. #M230) to enable the advantages of CHO-endogenous RVLPs for downstream process development. Each kit includes a vial of RVLP stock solution, a 96-well plate for sample analysis, RNA-extraction, and qPCR reagents, and a well-controlled RNA standard for accurate and reliable RVLP quantification. By following the kit’s protocol, scientists can detect as little as 103 RVLP/mL, enabling LRV of ~5.0 to be determined. Each kit contains 2.0 mL of RVLP stock solution at a concentration of 1010 RVLP/mL, sufficient for spiking up to 200 mL of load material to 1% (v/v) and conducting analysis on 23 samples in triplicate in under 24 hours. An RT-qPCR instrument is required along with standard laboratory equipment. Minimal experience with RNA extraction and qPCR protocols is required. Compatible purification steps include virus filtration and protein-A, AEX and CEX, MMC, hydrophobic-interaction, and size-exclusion chromatography methods. The MockV RVLP kit provides LRV accuracy to within ±0.5 for those modes of separation compared with traditional viral-clearance studies using XMuLV model virus.
With Cygnus’s BSL-1–compatible viral clearance kits, you can quantify viral clearance for downstream process steps easily and economically in your own laboratory and on your timeline. Why delay until late-phase clinical manufacturing before testing whether your downstream purification process steps provide sufficient viral clearance? Instead, you can gain actionable insights throughout process development and characterization. Instead of relying solely on a CRO-provided model retrovirus (XMuLV), downstream groups can spike and assess removal of the original retrovirus particle of regulatory concern in their own laboratories.
References
1 Lieber MM, et al. Mammalian Cells in Culture Frequently Release Type C Viruses. Science 182(4107) 1973: 56–59.
2 Lubiniecki AS, May LH. Cell Bank Characterization for Recombinant DNA Mammalian Cell Lines. Dev. Biol. Stand. 60, 1985: 141–146.
3 Anderson KP, et al. Endogenous Origin of Defective Retrovirus-Like Particles from a Recombinant Chinese Hamster Ovary Cell Line. Virology 181(1) 1991: 305–311.
4 CPMP/ICH/295/95. ICH Q5A: Quality of Biotechnological Products — Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. International Council for the Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 4 March 1997; http://www.pharma.gally.ch/ich/q5a029595en.pdf.
5 Lie YS, et al. Chinese Hamster Ovary Cells Contain Transcriptionally Active Full-Length Type C Proviruses. J. Virol. 68(12) 1994: 7840–7849.
6 De Wit C, Fautz C, Xu Y. Real-Time Quantitative PCR for Retrovirus-Like Particle Quantification in CHO Cell Culture. Biologicals 28(3), 2000: 137–148
7 Zhang M, et al. A Novel, QPCR Based Approach to Measuring Endogenous Retroviral Clearance By Capture Protein A Chromatography. Biotechnol. Bioeng. 102(5) 2009: 1438–1447.
8 Zhang, M, et al. Quality By Design Approach for Viral Clearance By Protein A Chromatography. Biotechnol. Bioeng. 111(1) 2014: 95–103.
9 Strauss DM, et al. Removal of Endogenous Retrovirus-Like Particles from CHO-Cell Derived Products Using Q Sepharose Fast Flow Chromatography. Biotechnol. Prog. 25(4) 2009: 1194–1197.
10 Strauss DM, et al. Strategies for Developing Design Spaces for Viral Clearance By Anion Exchange Chromatography During Monoclonal Antibody Production. Biotechnol. Prog. 26(3) 2010: 750–755.
11 Pan C, et al. Characterizing and Enhancing Virus Removal By Protein A Chromatography. Biotechnol. Bioeng. 116(4) 2019: 846–856.
12 ICH Q5A(R2) (Draft). Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. US Fed. Reg. 87(218) 2022; https://database.ich.org/sites/default/files/ICH_Q5A%28R2%29_Step2_draft_Guideline_2022_0826.pdf.
David Cetlin is senior director, MockV products, at Cygnus Technologies; [email protected]. Alla Zilberman is vice president, technical marketing at Cygnus Technologies; [email protected].
To learn more about Cygnus MockV RVLP and MockV MVM kits, visit https://www.cygnustechnologies.com/products/viral-clearance-kits/mockvmvm-kit.html.
You May Also Like






