Enhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with SuppliersEnhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with Suppliers
September 23, 2014
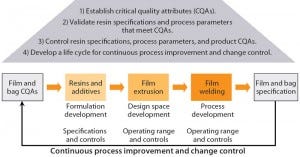
Figure 1: Material science and quality by design (QbD) approach for development of Flexsafe bags
Growing adoption of single-use bags in commercial production of biopharmaceutical drugs raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, polymer scientists and biologists at Sartorius Stedim Biotech have followed a stringent material science and quality by design (QbD) program to develop a completely new polyethylene film and to achieve consistent performance of new Flexsafe bags for all bioprocess steps and applications.
Reliability of the supply chain for Flexsafe bags is based on partnerships and agreements with suppliers and our complete understanding and control of the bag manufacturing process — from resin and film extrusion to final sterile bioprocessing bags. Consistent product performance is ensured by establishing specifications, traceability, and control of resins and additives as well as definition of a design space for film extrusion.
Assurance of supply relies on a long-term contract and partnership with our film supplier and its large extrusion capacity, which allows us to absorb the projected double-digit growth for single-use solutions. In addition, establishment of resin specifications rather than simply using resins based on their trade names provides robust change control and facilitates change management. Our company can rapidly qualify equivalent resins sourced from preferred resin suppliers should one supplier discontinue or introduce changes to necessary raw materials. Business-continuity planning is finally addressed through a mix of already available redundant equipment and manufacturing locations, qualification of back-up equipment, and maintenance of safety stocks for resins and film rolls.
Facing New Challenges
Single-use bioprocessing bags are widely adopted today by established biopharmaceutical companies for development and commercial production of biological drug products and vaccines. Single-use technologies offer safer, cheaper, faster, smaller, and “greener” biomanufacturing options and meet the main challenges of the biopharmaceutical industry for capacity adaptation, cost savings, risk mitigation, and process transfer. For smaller biotechnology companies, single-use technology also offers a fast and economic approach to rapidly implement flexible manufacturing capacity (1).
The technical and economic benefits of using single-use technologies are well known, and the biotechnology industry is broadening their adoption into all bioprocess steps such as cell culture, storage, shipping,
mixing, freezing and thawing, final filling, and sampling (2). In the past, most of the single-use bags were used for noncritical media and buffer storage applications. Those bags did not necessarily address fully the more critical quality demands of biopharmaceutical process intermediates, bulk drug substances, and final drug products.
The growing use of disposable bags in all critical process steps — both for process development and commercial production of high-quality biological drugs — raises new challenges for bag suppliers. Modern single-use solutions need improved quality, robustness and assurance of supply; more robust change control; and well-established business continuity plans (3).
A Partnership, Material Science, and QbD Approach
To meet those new challenges for a more robust quality of single-use bags, Sartorius Stedim Biotech developed a new, proprietary, PE S80 film in partnership with Südpack, our film manufacturer. That new film is the core component of our new Flexsafe family of single-use bags for all bioprocess applications. Through our collaboration with Südpack, we have established direct contacts, trust, and long-term relationships with selected industry-leading suppliers of plastic resins. Direct access to (and the openness on the part of) our raw-material suppliers provides an unprecedented level of information on the initial resin and additive package formulation. That is the central part of our new strategy to achieve complete control over our entire supply chain, from raw materials to finished products. Our resin suppliers support our assurance-of-supply strategy through their open mind-set and strong understanding about (and contribution to) risk-analysis and change-control concepts.
The partnership with Südpack, open attitude, and long-term relationship with our resin suppliers have allowed us to combine material science and QbD expertise to achieve a complete understanding and control of our manufacturing process from the initial raw materials to the final bioprocessing bags.
We selected our resin–additive package and processing parameters for the S80 film after testing several raw materials and film compositions to identify one with the best physical and mechanical properties and the most consistent cell growth performance (5, 6). The film specification was established to enable reproducible manufacturing of Flexsafe bags that are suitable and scalable for all upstream, downstream, and aseptic bioprocessing steps, including cell culture, storage, shipping, mixing, freezing, thawing, and filling applications. Key user requirements cover, for example, consistent cell growth, biocompatibility, purity, robustness, gas barrier properties, cleanliness, and sterility of final single-use Flexsafe bags.
The biocompatibility of films is normally assessed using conventional USP chapter <87> and chapter <88> in vitro and in vivo biological reactivity tests. For the new Flexsafe bags, a standardized cell-growth assay was used at the design stage to optimize the resin, the additive package, and the film formulation and to ensure their compatibility with a broad range of cell lines (5). Cell-growth testing also allowed us to determine the operating ranges for extrusion, welding, and γ-irradiation processes and establish specifications and critical process controls (4).
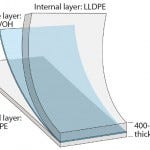
Figure 2: PE S80 multilayer film structure of
the new Flexsafe bag family (patent pending)
Purity of films and bags is characterized by their extractable and leachable profiles. It is determined by the nature of the polymer, the amount and nature of additives used to enable processing, and the processing parameters themselves. With a well-known and well-controlled resin, our polymer experts can thoroughly characterize the extractable profiles of Flexsafe bags and ensure their reproducibility. End users can be assured that their own initial extractable and leachable qualification work and data will remain valid every time they operate single-use bioprocesses using Flexsafe bags.
The robustness and barrier properties of Flexsafe bags are obtained by combining thickness, strength, and flexibility for each layer of S80 film. Together with application knowledge, our resin, film, and bag expertise allowed us to develop a 400-μm thick structure that offers outstanding robustness of film, welds, and bags for all bioprocessing applications in upstream production, downstream processing, and final filling.
The S80 film used in Flexsafe bags comprises a polyethylene (PE) contact layer and a coextruded backbone structure of PE, ethylene vinyl alcohol (EVOH), and PE. The formulation of resins and additives for both the contact and backbone layers are completely known, and the extrusion process parameters are controlled within established process-parameter ranges. That ensures lot-to-lot consistency of all critical quality attributes (CQAs) of Flexsafe bags: cell growth, robustness, barrier properties, and extractable profiles.
Enhanced Quality Assurance and Assurance of Supply
The entire supply chain for single-use bags is complex and requires material science and film expertise to achieve consistent quality attributes of film; bags; and final, sterile, single-use assemblies.
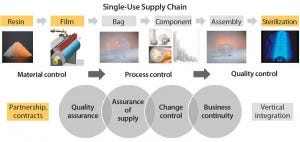
Figure 3: Overall supply chain for single-use bioprocessing bags
First, the key aspect of quality assurance consists in collaboration with manufacturers of resins and additive packages used for extrusion of polymer films. Working directly with suppliers of raw materials and plastic resins is of paramount importance in establishing specifications, operating ranges, and process controls and obtaining full understanding and traceability of the initial resin and additive package used for making a film.
Second, robust product quality necessitates a full understanding of the complete process, definition of operating ranges, and implementation of multiple controls to guarantee lot-to-lot performance of films, bags, and single-use bag assemblies. Such an overall knowledge and understanding of the entire process — from resin to final product — can be achieved only through strong relationships and partnerships among single-use bag manufacturers, raw-material suppliers, and film manufacturers.

Figure 4: Global Sartorius Stedim Biotech supply chain and manufacturing infrastructure for
single-use bags
Our knowledge about the formulation of the resins, additives, and catalysts as well as the critical process parameters (CPPs) for film extrusion makes it possible for us to understand which CPPs influence the CQAs of our final product. This QbD approach helped us develop more detailed resin specifications for both the contact layer and film backbone.
Also, our detailed knowledge regarding the formulation of compounds used in manufacturing our films enables us to establish consistent and relevant extractable profiling, data reporting, and leachable validation support. Knowing the compounds in the films helps us interpret our extractable data very precisely and accurately. This approach has been implemented to consolidate the supply of our existing S71 ethylene vinyl acetate (EVA) film used for making Flexboy and Celsius bags and in development of our new S80 PE film used for the new Flexsafe bag family.
Assurance of Supply Through Partnership and Long-Term Supply Agreements with Resin and Film Suppliers: Supply assurance for Flexsafe bags is guaranteed first by our long-term supply contracts established with different resin suppliers and with Südpack, our film manufacturer. We have a 10-year supply agreement in place for the S80 PE film and a last-time buy option for a minimum of two years of resin demand in case of changes or discontinuation. We also benefit from Südpack’s large, state-of-the-art, film-extrusion capacity, which will be required to sustain an expected yearly double-digit growth of the demand for single-use bioprocessing bags. Here again, complete control of our supply chain and manufacturing processes — from raw materials to final assemblies — is critical to offering both quality and assurance of supply.
Robust Change Control Procedures
Our long-term contracts with resin and film suppliers involve six months’ notification for changes in raw materials and/or manufacturing processes of the resin and two years’ notification for changes to the film. In addition, we have a last-buy time option of unchanged material for meeting two years of resin demand.
Even more important is establishment of resin specifications instead of using resins by their trade names. This provides robust resin change control and facilitates change management. With our established specifications and understanding of the resin’s CQAs, we can rapidly qualify an equivalent resin with our preferred resin suppliers. Releasing and controlling resins against specifications — not trade names — enhances the reliability of film quality, facilitates change management, and improves long-term assurance of supply with fast implementation of alternative resins in case of change or discontinuation of some raw materials. And the design space we have established for the film-extrusion process provides a fast and easily qualified implementation of new extrusion equipment should a change or a capacity ramp-up be required.
Business Continuity Planning
The terms business continuity planning and assurance of supply often, incorrectly, are used interchangeably.
Assurance of supply reflects our capability as a leading single-use supplier to consistently provide products according to defined specifications and quality requirements under normal operating conditions. This is the result of our long-term contracts, our large manufacturing capacity for all process steps, and the control of our entire process from raw materials and films to final sterile products.
Business continuity planning is required to cover unexpected and unlikely events such as raw-material discontinuation or manufacturing shut-down due to earthquake, fire, flood, storms, or other potential disasters. This is best achieved by ensuring that every critical process step can be performed with at least two different pieces of equipment and/ or at two different manufacturing locations. When neither redundant process equipment nor an alternative production site is available, another alternative is to maintain safety stocks that cover demand for the time that would be necessary to build and qualify alternative equipment or a new production line.
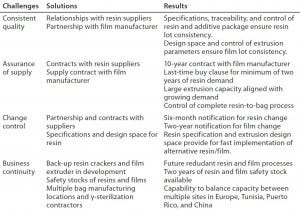
Table 1: Global approach to quality, assurance of supply, change control, and business continuity
Sartorius Stedim Biotech is currently qualifying a backup resin cracker and a second film extruder at our suppliers and partners to further enhance our business continuity planning. In the meantime, we maintain at least two years of safety stock for resins and films. Multiple bag-making equipment and assembly lines are installed in multiple manufacturing locations in France, Tunisia, and Puerto Rico to ensure business continuity planning for our final bag assemblies. We also have contracted multiple gamma-irradiation sterilization sources and suppliers worldwide to ensure final sterility assurance.
Conclusion
Sartorius Stedim Biotech has been working over the past few years on a strategy to enhance quality, supply assurance, and change control for its single-use bioprocessing bags and intelligent single-use solutions consisting of bags, filters, tubes, connectors, sensors, automation, and hardware. This strategy is based first on partnerships and long-term contracts with suppliers, which ensure control of raw materials and films as well as extension of resin and film-extrusion capacity. Second, it is based on the continuous expansion of our own manufacturing capacity in multiple sites to allow flexible business contingency planning. This strategy has been applied both in development of our new S80 polyethylene film and Flexsafe bag family and in strengthening the supply chain of our existing S71 film made of an EVA contact layer and used for manufacturing Flexboy and Celsius bags.
Establishing resin specifications and film-extrusion design space for controlling the entire manufacturing process provide long-term supply assurance, consistent product performance, and robust change control. Flexible business continuity planning is guaranteed by establishing back-up equipment for resin cracking and film extrusion, multiple bag manufacturing locations, and safety stocks of resins and extruded film rolls. In addition to the current EVA and new PE bags, we also control the manufacture of other critical components such as filters, connectors, tubing, and sensors and assemble them into our single-use solutions at multiple manufacturing locations worldwide.
References
1 Duffy K. Introduction of Technical Innovations to Improve Sterility Assurance in Vaccines Production. BioProcess International European Conference and Exhibition, 18 April 2012.
2 Large P. MCC Fujifilm Billingham. Single-Use Workshop, 19 Sept 2013. PDA UK Chapter; www.pda.org/Chapters/Europe/ United-Kingdom.aspx.
3 Langer E. Ninth Annual Report and Survey of Biopharmaceutical Capacity and Production. BioPlan Associates, Inc.: Rockville, MD, 2011.
4 Imseng N, et al. Towards a Standardized Biological Test for the Identification of Cell Growth Influencing Leachables (poster). ESACT 2013, 23–26 June 2013, Lille, France.
5 Fenge C, et al. Consistently Superior Cell Growth Achieved with New Polyethylene Film Formulation. BioProcess Int. 12(8) 2014: S34–S37.
6 Vachette E, et al. Robust and Convenient Single-Use Processing with Superior Strength and Flexibility of Flexsafe Bags. BioProcess Int. 12(8) 2014: S38–S42. c
Corresponding author Jean-Marc Cappia is vice president of marketing for fluid-management technologies ([email protected]); Elisabeth Vachette is global product manager for Flexel and Flexsafe (elisabeth.vachette@ sartorius-stedim.com); Carole Langlois is senior global product manager for Flexboy, ATS, and DDS at; and Magali Barbaroux is R&D director at Sartorius Stedim Biotech France. Heiko Hackel is vice president of global sourcing for Sartorius Group at Sartorius Stedim Biotech, August-Spindler- Straße 11, DE-37079 Goettingen, Germany.
You May Also Like





