Improving Immune Responses To Treat Breast Cancer Strategies for Developing Therapeutic Cancer VaccinesImproving Immune Responses To Treat Breast Cancer Strategies for Developing Therapeutic Cancer Vaccines
The American Cancer Society (ACS) identifies breast cancer as the second leading cause of cancer death among women in the United States (1). About 13% of American women will be diagnosed with invasive breast cancer during their lives, and 310,720 patients are expected to receive new diagnoses in 2024. Mortality rates have decreased steadily since the late 1980s as screening methods have improved and become more accessible. When tumors are detected early, patients’ five-year survival rate is 99%. But if tumors have spread to lymph nodes and other nearby tissues, that rate decreases to 85%. Prognoses become much less optimistic in cases of metastatic disease (when tumors have spread to distant organs), with five-year survival rates falling to 27%. Considering the prevalence of breast cancer and the difficulty of treating advanced cases, patients still have significant need for effective treatments.
Therapeutic vaccines could provide hope for many patients with advanced breast cancer and other solid-tumor indications. Such drugs are designed to enhance and, in some cases, activate anticancer responses by presenting cancer antigens to patient immune cells (2). Products can be based on either tumor-associated antigens (TAAs), which are overexpressed in cancers but also appear in some healthy cells, or tumor-specific antigens (TSAs), which are expressed in cancer cells only (3). The latter class includes neoantigens, peptides, and proteins that derive from point mutations, insertions, deletions, and other genetic changes in cancer cells (3). By leveraging immunization, drug developers intend to induce tumor destruction while reducing chances for adverse effects that are associated with other treatment modalities (2). In principle, a vaccination-based approach also could prime patient immune systems for long-term antitumor memory (2).
Although therapeutic cancer vaccination has been studied for more than a century, it has been difficult to translate into effective products (4). Oncological barriers include the trickiness of tumor microenvironments (TMEs), which are notorious for resisting, suppressing, and evading immune detection. Drug developers also must negotiate the difficult terrain of antigen identification, an activity complicated by heterogeneity in both TMEs and patient immune systems. It is no surprise, then, that the US Food and Drug Administration (FDA) has approved only two cancer vaccines to date: Provenge (sipuleucel-T, Dendreon Pharmaceuticals), an immunotherapy for hormone-refractory prostate cancer, and T-VEC/Imlygic (talimogene laherparepvec, BioVex/Amgen), a modified oncolytic herpesvirus for treatment of advanced melanoma (5, 6).
Interest in therapeutic cancer vaccines is surging, however. Saxena et al. explain that technologies for multiomic analysis have increased in sophistication and accessibility, facilitating tumor-antigen discovery and increasing researchers’ understanding of native immune responses (7). Options also are proliferating for prompting immune-system recognition of TAAs and TSAs. Kaczmarek
et al. noted in 2023 that clinical trials were underway for several therapeutic-vaccine modalities, including candidates based on viruses and bacteria that are genetically modified to express antigens of interest (2). The same is true for human cells, including induced pluripotent stem cells (iPSCs), dendritic cells (DCs), gene-modified cells from established cancer lines, and even genetically manipulated cells from a patient’s tumor (2). In some cases, developers are administering antigenic peptides, monoclonal antibodies (mAbs), or messenger RNA (mRNA) encoding for the requisite antigens, with lipid nanoparticles and exosomes under investigation as delivery systems (2).
To learn about immunization-based treatments for breast cancer, I spoke with William V. Williams, who is president and chief executive officer (CEO) of BriaCell Therapeutics Corp. The company leverages an established cancer cell line to develop gene-modified cell therapies, focusing initially on treatment of metastatic breast cancers. As of February 2024, the company’s Bria-IMT (SV-BR-1-GM) candidate had reached pivotal phase 3 clinical trials (8). Therein, the candidate will be evaluated as a monotherapy and in combination with Incyte Corporation’s retifanlimab, a checkpoint inhibitor. Williams explained how administering genetically engineered, replication-incompetent cancer cells to patients could stimulate effective immune responses directly and indirectly. He also described advantages that cell-based immunization approaches could have over those based on peptide neoantigens and mRNA. Of particular interest is that engineered cancer-cell lines eliminate time and resource burdens associated with neoantigen identification. Thus, patients could quickly receive off-the-shelf treatments for rapidly progressing diseases.
A medical doctor (MD) and fellow of the American College of Physicians (FACP), Williams has more than 35 years of experience in medical research and biopharmaceutical development. Before joining BriaCell in 2016, he served as vice president of exploratory development at Incyte and vice president of clinical pharmacology and experimental medicine at GlaxoSmithKline. As head of rheumatology research at the University of Pennsylvania, Williams organized major research studies in receptor biology and helped to usher candidate DNA vaccines for cutaneous T-cell lymphoma into clinical trials. He holds bachelor of science degrees in chemistry and biotechnology from the Massachusetts Institute of Technology (MIT) and an MD from Tufts University School of Medicine.
Treating an Intractable Cancer
Which factors complicate the treatment of metastatic breast cancer? Immunotherapy works well against cancers with high mutational burdens and when patients’ bodies have already generated immune responses to tumors. Think of an immune response as a car: Immunotherapies tend to “take the foot off the brakes” of the response, but if the car is stationary and has no accelerator pedal, then the therapy will not move the car forward. Breast cancer typically does not have a high mutational burden, so it is not especially immunogenic. For instance, biopsied tissues show few tumor-infiltrating lymphocytes. As a result, such cancer is not amenable to immunotherapy.
One exception in terms of the mutational burden is triple-negative breast cancer (TNBC). In such cases, tumor cells lack estrogen and progesterone receptors and do not express human epidermal growth factor receptor 2 (HER2). Thus, tests for those three kinds of proteins all yield negative results. The high tumor-mutational burden in TNBC can elicit an immune response, so checkpoint inhibition, in combination with chemotherapy, is an approved therapeutic modality. But only about 20–30% of advanced breast cancers are TNBC, and that form is particularly aggressive. Even when patients receive both chemotherapy and immunotherapy, response rates to therapy fall well below 50% (1).
Early detection of breast cancer facilitates treatment. Emerging tumors can be excised relatively easily, and patients tend to have good prognoses. That is why doctors encourage mammograms and other screening methods. Still, over 43,000 women die of breast cancer each year in the United States (1). If a cancer has spread before it can be diagnosed, then subsequent treatment tends not to be curative. Some patients survive metastatic breast cancer, but many do not.
How might a vaccination strategy improve clinical outcomes for advanced breast cancer? As I mentioned, such cancers generally do not face challenges from preexisting immune responses. Thus, checkpoint inhibitors will be ineffective. But vaccination can generate immune responses against cancer cells. A better term in this context is immunization. People tend to think of vaccines as prophylactics. In our case, immunization is targeted and therapeutic rather than prophylactic.
Researchers have explored several immunization approaches over the years, including recombinant proteins and peptides. Today, many companies also are developing neoantigen peptides. First, clinicians screen a patient’s tumor to identify which neoantigens are expressed. Those peptides are synthesized ex vivo, then administered to the patient to produce an immunizing effect. Obviously, such immunotherapies are not off-the-shelf drugs.
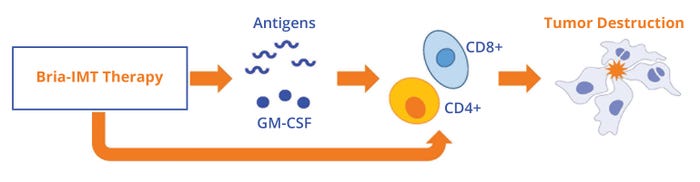
Figure 1: Bria-IMT cell therapy encourages tumor destruction by secreting granulocyte-macrophage colony-stimulating factor (GM-CSF) and by expressing tumor-associated antigens for recognition by CD4+ and CD8+ T cells. The therapy also activates CD4+ and CD8+ T cells directly (https://briacell.com/briaimt).
RNA-based vaccines present another option. They similarly target neoantigens. But instead of producing peptides for administration, RNA vaccines present cells with a genetic sequence encoding for neoantigen proteins. In other cases, developers such as BriaCell are using cell-based immunization approaches.
How would you describe your company’s
Bria-IMT therapy and how it fits into the cancer-immunization paradigm? It is a cellular immunotherapy based on a breast cancer cell line that we have genetically modified to produce and secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), which is an immunostimulator. Clinicians inject the therapy into the skin of a patient’s upper back and thighs to access draining lymph nodes.
In patients, the therapy performs at least three functions (Figure 1) (9). First, it expresses breast cancer antigens, which are taken up by dendritic cells in the skin, processed, and presented to CD4+ and CD8+ T cells in the context of human leukocyte antigen (HLA). Patient T cells then can recognize the antigen–HLA complex on tumors and subsequently destroy those cells. Second, the therapy secretes GM-CSF to boost dendritic-cell responses. Those two functions are similar to those of other cellular cancer vaccines. A third feature is unique to our therapy: Bria-IMT cells can serve as antigen-presenting cells (APCs), stimulating CD4+ and CD8+ T cells directly and thus increasing tumor destruction.
Producing a Whole-Cell Cancer Vaccine
How is the Bria-IMT therapy produced? We have already finished the genetic-modification component for the product, and the base cancer cell line has been stably transfected. So first, we expand the cell line in good manufacturing practice (GMP) conditions, harvest the cells, and then irradiate them so that they are replication incompetent. The cells are cryopreserved to ensure their stability. Currently, frozen cells are stable for five years. From there, it is an on-demand, off-the-shelf approach. We ship cells overnight to a clinic, where they are thawed and administered.
How amenable is the base cell line to gene modification? Is the transfection process difficult to perform? The process for our Bria-IMT product is straightforward. Our team has been working with different cancer cell lines for a second generation of therapies. Those lines have differed somewhat in their amenability to gene editing, but we have found that lentiviral transduction generally works well for our purposes.
Cells are tricky. Sometimes you try to insert a gene sequence, and they “spit it out.” They will not express the desired proteins or show suppressive expressions. Many activities go into finding the best possible combination. But again, we have found that lentiviral transduction is a good vehicle for gene transfer into cancer cells.
Does the Bria-IMT therapy have specific downstream requirements? In a way, making the Bria-IMT product is easier than the process for making a mAb. Recombinant proteins are produced during cell culture, so the supernatant must be harvested and purified. We simply harvest, wash, irradiate, and freeze our cells. After irradiation, the cells can no longer divide, but more than 90% of them are still alive and able to perform their immunotherapeutic functions in vivo.
What are the most difficult aspects of producing the Bria-IMT therapeutic? Considering that we have already developed a stably transfected cell line, release testing has become the most arduous part of our process. Regulatory agencies require many different tests of product identity, purity, and sterility, as well as assays for mycoplasma and adventitious-virus contamination. Drug developers must undertake a lot of work to show the FDA that their products have consistent characteristics. Release testing also can take a couple of months to perform. Thankfully, that is a tractable problem; it is a question of how effectively teams can execute the testing.
What are you learning about manufacturability and scale-up now that the Bria-IMT therapy has reached phase 3 clinical studies? We believe that the therapy can be manufactured scalably. We have yet to reach the commercial stage, at which production volumes increase significantly. The best scalability strategy to use remains somewhat of an open question. But we have yet to encounter clear scalability issues. That success is due in part to our having a stably transfected cell line that shows good genetic stability over time.
Comparing Immunization Approaches
You mentioned peptide- and RNA-based cancer vaccines. Many companies are jumping on the mRNA bandwagon, touting the straightforward development and production of such drugs. Considering such modalities, why might cell-based immunization still be a valuable approach for cancer treatment? One factor is that RNA-based approaches — at least how companies use them now — do not provide off-the-shelf solutions. Clinicians must identify the antigens in a given patient, synthesize and purify the requisite RNA in a GMP setting, and finally treat that particular patient. Our approach at BriaCell yields an allogeneic product that can be made readily accessible.
Another advantage of Bria-IMT cells and other products based on cancer-cell lines is that they express not one, but multiple kinds of TAAs and TSAs. Peptide- and RNA-based approaches often leverage a specific neoantigen to immunize patients, but cancer cells have an incredible ability to turn off genes and intercellular signaling pathways that would otherwise hinder their chaotic activity. Immunizing patients against several antigens simultaneously will broaden their immune responses, making it difficult for tumors to evade detection.
Our cells also express antigens that have undergone posttranslational modifications (PTMs). The biopharmaceutical industry has yet to appreciate the importance of PTMs in an immunization context, I believe. My training is in immunology, autoimmune disease, and rheumatology. So I often think about proteins that have undergone citrullination, a PTM during which arginine converts to citrulline through substitution of an oxygen molecule for a nitrogen molecule. Chemically, the change is simple, but the resulting antigen is considerably different than what you had before. Citrullinated proteins are implicated in rheumatoid arthritis (RA). The antibodies that RA patients generate against such proteins cause the disease. But autoimmune disease and cancer sometimes seem chiral to each other: The problem underlying autoimmune disease raises opportunities for cancer treatment. Conditions such as RA demonstrate that posttranslationally modified antigens can be immunogenic. And if a tumor expresses such antigens, then immune responses can be generated against it.
What other factors must BriaCell consider as it develops new therapeutic cancer vaccines? In our case, we must remember that the mechanism for directly stimulating an immune response depends on HLAs, which present pathogenic peptide antigens to T cells. T-cell receptors are designed to recognize entire antigen–HLA complexes. However, HLA molecules are polymorphic, meaning that different patients can have cells with different HLA markers. During clinical studies for our Bria-IMT candidate, we noticed that patients whose HLA type matched that of the therapy were more likely to have clinical benefit than were patients with mismatched HLA types. We have shown in published experiments that our cell lines directly stimulate T-cell clones in an HLA-restricted, antigen-specific way (10). So if we can produce a cell therapy that matches a given patient at the HLA type, then the therapy will be more likely to generate a positive clinical outcome.
That insight has informed our drug-development pipeline. We have genetically modified our cell lines further so that they express different HLA types. We call those cell lines “Bria-OTS” therapeutics to emphasize that they are off-the-shelf products (Figure 2). With 15 HLA types expressed across four distinct cell lines, we can match >99% of patients. Thus, making such changes to our base cell line enables us to personalize our therapies while preserving their status as off-the-shelf products. That combination could be an innovative approach to cancer immunotherapy. Currently, our Bria-OTS candidate has entered phase 1–2 clinical trials.
We also have modified our cells so that they can stimulate naive T cells. Thus, the therapies do not simply boost existing immune responses, but rather initiate responses against cancer antigens. That next generation of therapies could be transformational for the oncology field at large: A series of genetically modified cell lines could be developed for different diseases — e.g., breast cancer, prostate cancer, lung cancer, melanoma, and so on (Figure 2, right).
Our goals, then, are to develop immunotherapies that are both personalized and off-the-shelf for cancer treatment and to have those therapies be capable of inducing — rather than just elevating — a potent immune response against a given patient’s cancer. Therapies that can achieve both of those goals could be highly effective and, in the long term, change how we think of cancer treatment.
References
1 Key Statistics for Breast Cancer. American Cancer Society: Kennesaw, GA, 2024; https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html.
2 Kaczmarek M, et al. Cancer Vaccine Therapeutics: Limitations and Effectiveness — A Literature Review. Cells 12(17) 2023: 2159; https://doi.org/10.3390/cells12172159.
3 Biswas N, et al. Designing Neoantigen Cancer Vaccines, Trials, and Outcomes. Front. Immunol. 14, 2023: 1105420; https://doi.org/10.3389/fimmu.2023.1105420.
4 Schroeder B. A Snapshot of Cancer Vaccine Development. MIT News 15 August 2023; https://news.mit.edu/2023/snapshot-cancer-vaccine-development-0815.
5 Anassi E, Ndefo UA. Sipuleucel-T (Provenge) Injection: The First Immunotherapy Agent (Vaccine) for Hormone-Refractory Prostate Cancer. Pharm. Ther. 36(4) 2011: 197–202; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3086121.
6 Ferrucci PF, et al. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers (Basel) 13(6) 2021: 1383; https://doi.org/10.3390/cancers13061383.
7 Saxena M, et al. Therapeutic Cancer Vaccines. Nature Rev. Cancer 21, 2021: 360–378; https://doi.org/10.1038/s41568-021-00346-0.
8 NCT06072612. Study of the Bria-IMT Regimen and CPI Versus Physicians’ Choice in Advanced Metastatic Breast Cancer. US Clinical Trials Database 31 October 2023; https://clinicaltrials.gov/study/NCT06072612.
9 Wiseman CL, Kharazi A. Phase I Study with SV-BR-1 Breast Cancer Cell Line Vaccine and GMCSF: Clinical Experience in 14 Patients. Open Breast Cancer J. 2, 2010: 4–https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3086121.11; http://dx.doi.org/10.2174/1876817201002010004.
10 Lacher MD, et al. SV-BR-1-GM, a Clinically Effective GM-CSF-Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Front. Immunol. 9, 2018: 00776; https://doi.org/10.3389/fimmu.2018.00776.
Brian Gazaille, PhD, is managing editor of BioProcess International; [email protected]. William V. Williams, MD, FACP, is president and chief executive officer of BriaCell Therapeutics Corporation, 2929 Arch Street, Third Floor, Philadelphia, PA 19104; [email protected]; 1-888-485-6340.
You May Also Like






