Vaccine Research and Development Infrastructure in the European Union: Establishing Support Through IntegrationVaccine Research and Development Infrastructure in the European Union: Establishing Support Through Integration

Developing novel medicinal products involves many processes and requires a number of different technologies and areas of expertise. Although large pharmaceutical companies can support in-house development and fill gaps by hiring contract development and manufacturing organizations (CDMOs), the cost, complexity, and scope of development are prohibitive for most academic or small- and medium-sized enterprises (SMEs). Such difficulties are amplified for vaccine developers, especially those working to treat vulnerable populations in low- and middle-income countries where profit potential is limited. A permanent vaccine research infrastructure (RI) in Europe could relieve developmental obstacles (1).
The COVID-19 pandemic highlighted the need for flexible vaccine-production capacity and operational excellence. Research innovations from university and SME professionals demonstrated the potential of mRNA vaccines (2). But COVID-19 mRNA vaccine successes have yet to translate to other public-health needs. Many academic institutions and biotechnology companies have few resources for advancing their discoveries into clinical development.
The Role of Vaccine RI
Distributed RIs could address infrastructural challenges and provide a sustainable solution that helps to coordinate science across Europe. RIs enable international consortiums of universities, research institutions, and SMEs to serve scientific communities, facilitate partnerships, and reduce duplicate efforts. They use a synergistic approach, providing a public-facing interface and improving integration among institutions.
In 2009, 14 European institutions formed the TRANSVAC network with support from the European Commission, offering free access to a full suite of scientific services to vaccine developers. Over the past 14 years, TRANSVAC has worked to address a number of challenges identified during its first years of operation (3, 4). The European Union (EU) Horizon 2020 program called TRANSVAC2 expanded upon the original program’s goals, uniting 26 partner institutions that offer more than 50 scientific services, including animal studies, adjuvant development, immunocorrelate analysis, and quality control (QC) (see “Partners” box).
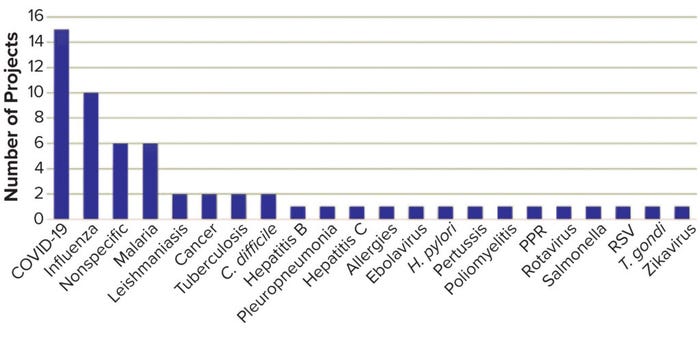
Figure 1: Targets of granted TRANSVAC2 projects.
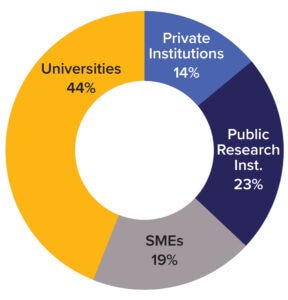
Figure 2: Overview of TRANSVAC2 user institutions (SMEs = small- and mediumsized enterprises).
TRANSVAC and TRANSVAC2 have combined to review 158 free-service applications and have approved 88 projects from 19 countries. Nearly a quarter (18) of those projects have involved services from multiple institutions. User programs have targeted a breadth of human and veterinary diseases (Figure 1). Although universities and research institutions are the majority of program users, the private sector accounts for 33% (Figure 2).
Program support has enabled drug candidates to advance to clinical trials. The MTBVAC Mycobacterium tuberculosis vaccine has progressed the furthest, advancing to phase 3 clinical trials after TRANSVAC2 provided critical animal studies (5). Nevertheless, several years will pass before program success is evident. TRANSVAC2 also has organized a free 14-course vaccinology class. More than 400 trainees have attended, helping to fill an important training gap in the European industry.
Prospects for a European Vaccine Infrastructure
In a report that assessed the sector’s needs in Europe, we asked experts to identify the most pressing challenges for vaccine development (6). Most respondents said that RI is needed to promote vaccine-developer access to scientific services, expertise, and funding.
Developing a vaccine candidate requires selection of an effective adjuvant for formulation with a given antigen. That process can be challenging for many reasons. In addition to formulation and immunogenicity considerations, adjuvants must be produced affordably at scale, have a strong regulatory track record, and be clear of intellectual property (IP) encumbrances. Vaccine developers often struggle to obtain adjuvants, expertise, and associated data despite efforts to facilitate access by groups such as the Vaccine Formulation Institute (VFI).
The preclinical pipeline presents further difficulties. Animal-model availability is limited, and sufficiently graded manufacturing material can be prohibitively expensive. Issues with manufacturing costs are amplified by the funding infrastructure. Although early research often is supported by public agencies, the private funding needed to support late-stage research is reportedly difficult to obtain in Europe.
Respondents to our survey indicated that even if a candidate vaccine enters clinical trials, regulatory expertise is insufficient among many academic and SME developers. Licensure-requirement expertise is critical in some countries, and some groups lack resources and training. That was evident from both our surveys and the use patterns of the TRANSVAC2 training modules. The European Infrastructure for Translational Medicine (EATRIS) course, Regulatory Aspects of Vaccine Development, drew some of the highest demand. Applicants represented research institutions, small biotechnology companies, and large pharmaceutical companies.
TRANSVAC2 is concluding as an independent EC-funded project. Program managers seek to leverage its expertise to become a sustainable RI for vaccine development. Although EC support enabled multiple institutions to integrate into a single-access platform, TRANSVAC2 is likely to need further sources of funding to support a viable long-term infrastructure. The European Strategy Forum on Research Infrastructures (ESFRI) might provide such support, having promoted and sustained many RIs. Forty-seven distributed scientific RIs appeared on the 2021 ESFRI Roadmap (7). However, reaching the destination marked on the map probably will take several years.
In the short term, TRANSVAC2 could form an independent infrastructure to offer contractual services or transition to a bioholding model, in which it supports promising vaccine candidates in return for a stake in the IP. Profits from successful bioholding projects would be reinvested, including into projects with low commercial potential but important health implications.
The next few years will demonstrate whether TRANSVAC’s efforts translate into long-term integration within the European vaccine sector. Ultimately, a permanent, distributed vaccine RI will empower researchers from academia and SMEs. It also will support TRANSVAC’s mission to advance needed human and veterinary vaccines.
Acknowledgments
This work received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 730964 (TRANSVAC2). We gratefully acknowledge the support of the entire TRANSVAC, TRANSVAC2, and TRANSVAC-DS consortiums and their contributions to the completion of these projects; https://www.transvac.org/training.
References
1 Plotkin S, et al. The Complexity and Cost of Vaccine Manufacturing — An Overview. Vaccine 35(33) 2017: 4064–4071; https://doi.org/10.1016/j.vaccine.2017.06.003.
2 Dolgin E. The Tangled History of mRNA Vaccines. Nature 597, 2021: 318–324; https://doi.org/10.1038/d41586-021-02483-w.
3 Leroy O, et al. Roadmap for the Establishment of a European Vaccine R&D Infrastructure. Vaccine 32(51) 2014: 7021–7024; https://doi.org/10.1016/j.vaccine.2014.08.014.
4 Medaglini D, et al. Innovation Partnership for a Roadmap on Vaccines in Europe (IPROVE): A Vision for the Vaccines of Tomorrow. Vaccine 36(9) 2018: 1136–1145; https://doi.org/10.1016/j.vaccine.2017.11.069.
5 Martín C, et al. MTBVAC, a Live TB Vaccine Poised To Initiate Efficacy Trials 100 Years After BCG. Vaccine 39(50) 2021: 7277–7285; https://doi.org/10.1016/j.vaccine.2021.06.049.
6 Jungbluth S, et al. A Gaps-and-Needs Analysis of Vaccine R&D in Europe: Recommendations To Improve the Research Infrastructure. Biologicals 76 2022: 15–23; https://doi.org/10.1016/j.biologicals.2022.02.003.
7 Strategy Report on Research Infrastructures: Roadmap 2021. European Strategy Forum on Research Infrastructures: Brussels, Belgium, 2021; https://roadmap2021.esfri.eu.
Corresponding authors William J. Martin ([email protected]) and Monika Slezak ([email protected]) are project managers, Romina Di Marzo ([email protected]) is communications and advocacy manager, Catarina Luís ([email protected]) is project and communications manager, Stefan Jungbluth ([email protected]) is head of business development, and Ole Olesen ([email protected]) is executive director, at European Vaccines Initiative, Heidelberg, Germany; [email protected]; 31-644-252830.
You May Also Like






