Biopharmaceutical Cleaning Validation: Experimental Evaluation of the Acceptable Exposure Limit for Inactive Therapeutic-Protein FragmentsBiopharmaceutical Cleaning Validation: Experimental Evaluation of the Acceptable Exposure Limit for Inactive Therapeutic-Protein Fragments
A typical sequence of pharmaceutical manufacturing and equipment cleaning operations in a multiproduct facility is shown in Figure 1. An important regulatory requirement for intercampaign cleaning — cleaning between batches of different products — is that the carryover of the previously manufactured active pharmaceutical ingredient (APIA) into a therapeutic dose of the subsequently manufactured product (Product B) must be acceptable from the standpoint of patient safety (1–5).
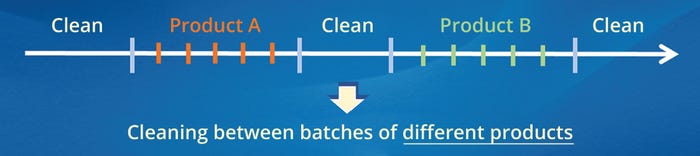
Figure 1: Typical sequence of pharmaceutical-manufacturing and equipment-cleaning operations in multiproduct equipment; the red and green bars represent batches of previously and subsequently manufactured products (A and B), respectively.
That requirement also applies to the preparation of newly manufactured and reusable medical devices. In either case, the criterion for patient safety is generally specified in terms of the acceptable exposure level (AEL) of the contaminant, which is APIA in this example (6–8).
Inactivation of Proteins During Cleaning and Sanitization: Biopharmaceutical cleaning and sanitization cycles are designed to expose product-contact equipment to high pH (>13) and temperature (60–120 °C) for several minutes. Under those conditions, therapeutic proteins and other biologicals degrade into pharmacologically inactive fragments (9–11).
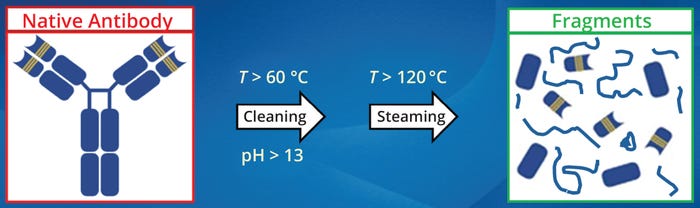
Figure 2: Degradation of therapeutic proteins during cleaning and steam sanitization; antibodies and other biologicals degrade into pharmacologically inactive fragments when exposed to high pH (>13) and temperature (60–120 °C) for several minutes (9–11)
Implications for Cleaning Validation: The inactivation of therapeutic proteins during cleaning and sanitization has important implications for cleaning validation. That is because the AEL, and therefore the acceptance limit (AL), for an active therapeutic protein is often below the capability of a given cleaning process. Additionally, the AL may also be below the limit of quantitation (LoQ) of analytical methods commonly used for biopharmaceutical cleaning validation, such as those based on measurement of ultraviolet (UV) absorbance and total organic carbon (TOC). Thus, these methods are often not adequately sensitive to accurately quantify an active therapeutic protein at its AL. For example, the AL for active therapeutic proteins is typically <0.1 µg/cm2, whereas the capability of most cleaning processes and the LoQs of commonly used analytical methods are typically >0.1 µg/cm2 (12). For those reasons, ALs for active therapeutic proteins often are not readily achievable.
Fragmentation and inactivation of proteins during cleaning and sanitization provide a practical and effective solution to such issues. The AEL, and therefore the AL, for inactive protein fragments (IPFs) is typically about two orders of magnitude greater than that of the active protein (1, 2, 8, 9). Thus, the AL for IPFs is generally well above the capability of most cleaning processes and the LoQs of common analytical methods; therefore, such ALs are readily achievable.
The higher AL for IPFs can be leveraged to simplify and streamline other aspects of cleaning validation. A higher AL generally results in higher surface and rinse recoveries and fewer cleaning validation failures, which in turn reduces the time and resources required to develop cleaning processes and introduce new products. Additionally, setting ALs based on the actual process residue or contaminants (e.g., the IPFs) rather than the active protein is more science-based, relevant, and reflective of the phenomenological aspects of the cleaning process. It is also consistent with the regulatory requirement that carryover of process contaminants into a subsequently manufactured batch must be justified from the standpoint of patient safety (3–6).
Evaluation of Protein Degradation and Inactivation: The experimental approach and analytical methods for evaluating degradation and inactivation of proteins during cleaning and sanitization have been discussed in the literature (10, 11, 13). In those experiments, process soil is exposed to full-scale cleaning and sanitization conditions. Experiments are performed at bench scale and are designed to simulate full-scale operating conditions that are least conducive, and therefore worst case, for protein degradation. The degree of degradation is evaluated by subjecting the process soil and untreated control material to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or some other analytical method that can evaluate the molecular weight distribution (MWD) of the protein fragments. The criterion for complete degradation is the absence of a distinct band at the molecular weight of the native protein (Figure 3). If the MWD data are inconclusive, a biological or other such activity assay can be used to evaluate the degree of inactivation (1, 2, 13, 14).
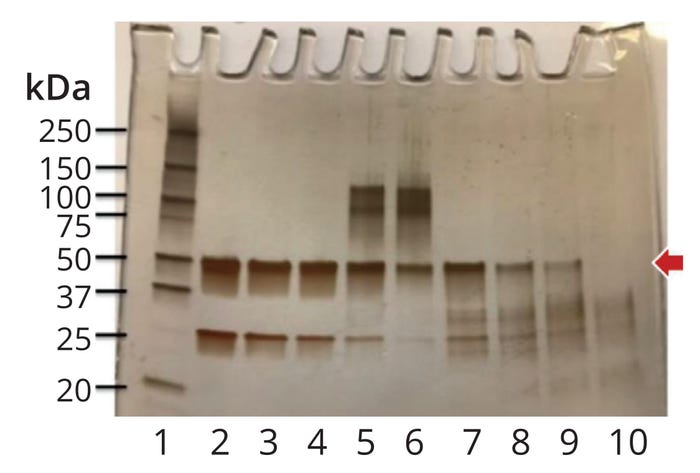
Figure 3: Evaluation of protein degradation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE); the red arrow indicates the molecular weight of the native protein (MWNP, 50 kDa in this case). Lane 2 shows the native protein (untreated control). The criterion for complete degradation is the absence of a distinct band at MWNP (Lane 10) (2).
Patient Safety and Product Efficacy: Patient-safety concerns can arise from the potential of degraded protein fragments to prime the immune system and elicit innate and adaptive immune responses (15, 16). Such responses can manifest as injection-site or infusion-related adverse reactions due to an inflammatory response. Degraded protein fragments also pose an immunogenicity risk because they can lead to formation of neoepitopes. Furthermore, the presence of protein fragments in a drug product can have an adjuvant-like effect that can modify the immunogenic potential of the therapeutic proteins (17). That can lead to a T-cell–dependent antibody response that can impact the safety and efficacy of the product.
Degradation of a therapeutic protein can greatly reduce the risk of target engagement and functional response; however, the intrinsic antigenicity of the degraded fragments can still exist. It is therefore necessary to evaluate the AEL of such fragments and use it to set safety-based ALs for cleaning validation.
Acceptable Exposure Limit of Inactive Fragments: The objective of this work was to determine the AEL for IPFs. Immunogenicity of the IPFs was evaluated by exposing them in vitro to human peripheral blood mononuclear cells (PBMCs) and subsequently measuring the immune response with human-immune-cell–based assays for cytokines. Immune-cell activation by the IPFs was assessed in the presence and absence of an active therapeutic protein. In the context of the sequence of operations in multiproduct equipment (Figure 1), the IPFs in our study represent degradants of the API that was previously manufactured in the equipment (APIA), and the active therapeutic protein represents the API in the subsequently manufactured product (Product B). The proposed methodology builds upon previously published approaches for evaluating an acceptable exposure limit for inactive protein fragments (1, 2, 7–9).
Materials and Methods
Cleaning and Steam Sanitization: Cleaning steps were performed in a temperature-controlled water-bath equipped with a linear shaker. Steam sanitization was performed in an autoclave with a liquid cycle. Stainless-steel plates (1 cm × 1 cm × 0.1 cm, hereafter called coupons) were used to simulate process-equipment surfaces. The coupons were fabricated from mechanically polished 316L stainless steel, the predominant material of construction in biopharmaceutical process equipment.
The coupons were exposed to cleaning conditions that were least conducive — and therefore worst-case — for protein degradation and inactivation: the shortest duration, the lowest temperature and concentration of cleaning solution, and laminar mixing. Those parameters were set to the low end of their respective operating ranges.
Antibodies: Human immunoglobulin G class 1 (IgG1)–based monoclonal antibodies 1 and 4 (mAb1 and mAb4) are commercially available as highly purified solutions. Two purified human IgG2 antibodies (mAb2 and mAb3) and a human IgG1 antibody (mAb1B) were supplied by Amgen’s Process Development group. Note that mAb1B is the same antibody as mAb1 but from a different source. All mAb solutions were highly purified and contained negligible amounts of protein aggregates. The rates of clinical immunogenicity were as follows:
• mAb1/mAb1B: >>10%
• mAb2: <1%
• mAb3: <7%
• mAb4: <1%.
Those mAbs all bind to different targets. Endotoxin levels of all samples were measured and found to be within the AL of the assay.
Preparation of IPF Samples: Coupons were precleaned with 1N sodium hydroxide (NaOH) at 70 ± 1 °C for five minutes. After the wash, the coupons were rinsed with deionized water for one minute and air-dried. Each coupon was then soiled with 0.2 mL of mAb1B, mAb2, or mAb3. The soiled coupons were then air-dried at 22 ± 1 °C for 72 hours, which was the desired postuse hold time for the process equipment (the desired time interval between the end of batch processing and the start of cleaning). Each soiled coupon was placed in a 50-mL glass vial containing 20 mL of the cleaning solution (0.5N NaOH) at 60 °C. The vials were then placed in a temperature-controlled water-bath equipped with a linear shaker. The temperature of the water-bath and the shaker speed were maintained at 60 °C and 10 cm/s for the duration of the wash. Under those conditions, flow across the surface of the coupon was laminar. After the wash, the protein–NaOH solution was titrated with phosphoric acid to neutral pH (7 ± 0.1) to minimize further degradation. The vials were then autoclaved at 121 °C for 10 minutes to simulate the steam sanitization step.
The samples and appropriate controls were analyzed by SDS-PAGE and a bioassay to determine the degree of degradation and inactivation, respectively. The bioassay results indicated that the antibodies were fully inactivated, and the absence of a distinct band at the molecular weight of the native protein indicated that the cleaning and sanitization conditions were effective in fully degrading the proteins into fragments (Figure 4, Lanes 4, 7, and 10).
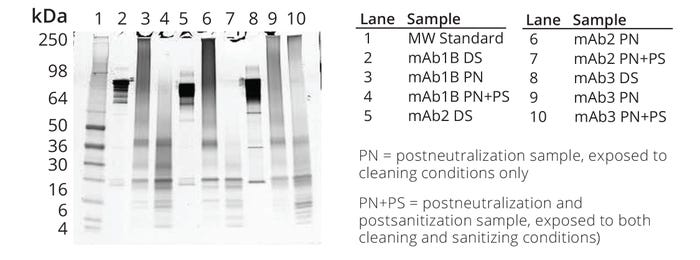
Figure 4: Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of untreated drug substance (DS), product that was exposed to cleaning conditions only, and product that was exposed to cleaning and sanitization conditions; sample load for the molecular-weight (MW) protein marker in Lane 1 and the protein samples in Lanes 2–10 were 10 μL and 1.2 μg per well, respectively. Samples in Lanes 4, 7, and 10 were evaluated for early- and late-phase immunogenicity.
In Vitro Assessment of Immunogenicity: In vitro PBMC assays based on human leukocyte antigen (HLA) haplotype are often used to understand sequence- and attribute-related immunogenicity risks of protein therapeutics and to ensure the selection and development of the least immunogenic candidate for clinical development (20–26). Such human-blood–derived assays ensure a real-time evaluation of the immune cells from human subjects representing HLA diversity. T-cell activation was assessed by measuring cytokine secretion, regulation of cell surface markers of activation, signal transduction events, and proliferation (Figures 5–7).
PBMC Preparation: PBMCs from human donors were supplied by Amgen’s Environmental Health and Safety group under local ethical practices. PBMCs were isolated in 8-mL BD Vacutainer Cell Preparation Tube (CPT) containers with sodium heparin (BD #362753), as described previously (20). Tubes containing whole blood were centrifuged at 1600×g for 30 minutes, forming a cloudy layer of PBMCs suspended directly above a polyester gel gradient. PBMCs were transferred to 15-mL conical tubes and washed in sterile phosphate-buffered saline (PBS) by centrifugation at 300×g for 15 minutes. The pellet was resuspended, and the cells were counted using a Cellometer Auto T4 cell-viability counter (Nexelcom). PBMC aliquots of 1 mL were diluted to 15 × 106 cells/mL in freezing media — fetal bovine serum (FBS) + 10% dimethyl sulfoxide (DMSO) — and frozen at –70 °C overnight prior to long-term storage in liquid nitrogen. Prior to experimental use, the PBMCs were thawed and diluted in growth media (89% RPMI 1640 medium, 10% FBS, 1% penicillin/streptomycin/l-glutamine), washed in sterile PBS, and counted.
PBMC Stimulation Assay: Human PBMCs from four to 12 donors (viability 82–92%) were plated at 2 × 106 cells/mL in a total volume of 200 µL in 96-well culture plates and challenged as previously described (20–25). The cells were challenged with the following protein fragments and active proteins:
• mAb1B fragments (10–1000 µg/mL) in the presence or absence of mAb1B (100 µg/mL)
• mAb2, mAb3, or mAb1B fragments (100 µg/mL) in the presence or absence of mAb1 (100 µg/mL)
• mAb2, mAb3, or mAb1B fragments (100 µg/mL) in the presence of mAb4 (100 µg/mL)
• mAb1 or mAb4 in the absence of fragments (100 µg/mL).
Negative controls treated with mAb1B excipients (buffer in which active mAb1B was formulated) or growth-media–treated cells only were assessed, as were positive controls treated with lipopolysaccharide (LPS) or phytohemagglutinin (PHA). Plates with challenged cells were incubated in a 5% CO2 incubator at 37 °C for 20 hours or seven days to evaluate early- and late-phase immune responses, respectively. Cell-derived supernatants were then frozen and stored at –70 °C for multiplexed cytokine assessment.
Multiplex Cytokine Analysis: Multiplex cytokine analysis was performed on culture supernatants using a Luminex assay and a MilliporeSigma Milliplex MAP kit (HCYTOMAG-60K) according to manufacturer instructions and as previously described (20–23). To normalize donor-to-donor variability, fold increases were calculated by dividing the cytokine concentrations in treated samples by the corresponding concentrations in the negative-control samples. The criterion for a positive immune response was a twofold or greater increase in any of the early- or late-phase cytokines assayed.
Results
The objective of this work was to determine the AEL of IPFs that form during cleaning and sanitization of process equipment. Proteins were inactivated under worst-case operating conditions — specifically, the shortest duration, the lowest temperature and concentration of cleaning solution, and laminar mixing. The studies were designed to determine whether relevant levels of IPFs have a potential risk for immunogenicity when they are carried over from the initially manufactured product (Product A) into the subsequently manufactured product (Product B).
The AEL of the IPFs was determined by exposing them in vitro to human PBMCs and subsequently assessing the induction of early- and late-phase immune responses using human-immune-cell–based assays for cytokines. The results were used to compare T-cell activation by the IPFs in the presence and absence of active therapeutic proteins. Inactive fragments were derived from three immunogenic proteins: mAb1B, mAb2, or mAb3. The active proteins, mAb1 and mAb4, had high and low immunogenicity, respectively. In the context of the sequence of operations in multiproduct equipment (Figure 1), the IPFs represent degradants of the API that was previously manufactured in the equipment (APIA); the active proteins represent the API in the subsequently manufactured product (Product B).
To assess the impact of IPFs in the absence of an active therapeutic protein, human PBMCs were challenged with inactivated fragments derived from a highly immunogenic antibody, mAb1B. The PBMCs were exposed to IPFs at concentrations of 10, 100, 200, 650, and 1000 µg/mL. Supernatants collected at 20 hours (early-phase immune response) and seven days (late-phase response) were analyzed using a Luminex assay based on 11-plex and 12-plex bead arrays, respectively (Figures 5a and 5b). The early-phase response to mAb1B fragments was associated with a slight increase in cytokine release above the negative-control samples that was above the assay threshold (black dotted line) but not above the biological threshold (red dotted line) (Figure 5a). The adaptive-phase response to mAb1B fragments was associated with a slightly higher increase in cytokine secretion — e.g., of interferon γ (IFNγ), interleukin 2 (IL-2), IL-5, and IL-13 — that exceeded the assay threshold (black dotted line) and, in a few cases, slightly exceeded the biological threshold (red dotted line). That slight response is likely due to the propensity of the protein itself to stimulate PBMC in these cell-based assays and to the overall variability of such biological assays rather than the immunogenic nature of the inactivated fragments. That conclusion is supported by the overall low level of cytokine secretion observed, the low numbers of donors responding (a maximum of one donor responded per sample type), and the absence of donors responding to the highest dose of mAb1B fragments tested (1000 µg/mL) (Figure 5b).
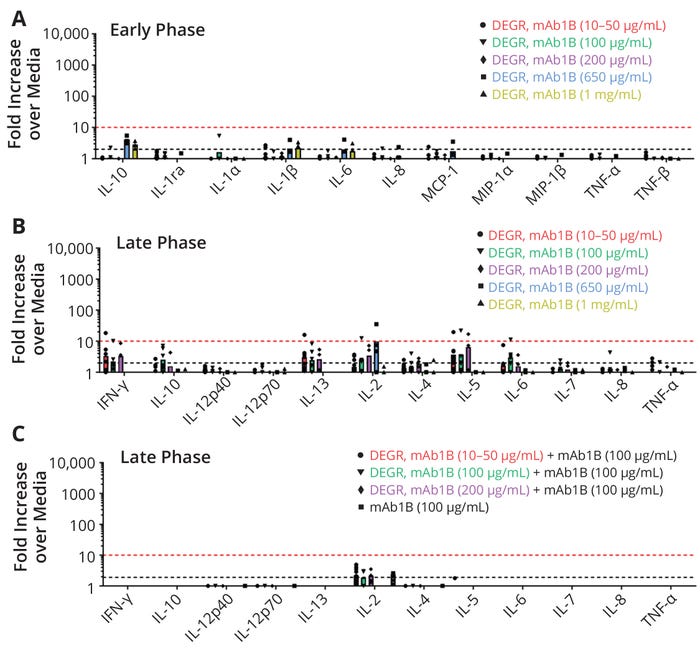
Figure 5: Immunogenicity risk assessment measured by multiplex cytokine release from human peripheral blood mononuclear cells (PBMCs) at early (20 hours) and late (seven days) immune-response phases; immunogenicity of mAb1B degradants (DEGRs) was evaluated at different concentrations between 10 and 1000 μg/mL. The y-axis plots fold increases over values from test media for each cytokine on the x-axis. The different shapes denote individual donor data, and the bars denote the average of all responding donors. The black dotted line represents the assay response threshold (≥2.0× over levels in the testing media), and the red dotted line is the biological threshold (≥10×). The number of donors for each set of PBMCs ranges from four to 12.
The impact of IPFs in a background of active therapeutic protein was also assessed. When inactivated fragments of mAb1B (10–200 µg/mL) were added to PBMCs in a background of active mAb1B (100 µg/mL), little to no cytokine secretion was observed. Almost none of the cytokines exceeded the assay threshold (black dotted line), and none of cytokines exceeded the biological threshold (red dotted line) (Figure 5c).
Additional studies were performed to assess if the immunogenicity of the degraded human therapeutic protein (HTP) has an impact on the immunogenicity risk of the inactive fragments. PBMCs were challenged with degraded fragments of three HTPs that had either low (mAb2 and mAb3) or high (mAb1B) rates of clinical immunogenicity (see "Sample Descriptions" at the bottom of the page). These fragments were tested in a background of either media only (Figure 6a), a molecule with a high rate of clinical immunogenicity (mAb1, Figure 6b), or a molecule with a very low rate of clinical immunogenicity (mAb4, Figure 6c). Figure 6a shows the late-phase profiles of PBMCs challenged with these therapeutic proteins at 100 µg/mL in a background of media. Inactivated protein fragments of mAb1B were associated with a slightly higher secretion of cytokines (IL-2, IFNγ, and IL-5) as compared with mAb2 and mAb3. That was above the assay threshold (black dotted line), but not above the biological threshold (red dotted line). A few donors were slightly above the threshold, which is likely due to variability of these cell-based biological assays.
When fragments from mAb2, mAb3, and mAb1B were added to PBMCs in a background of active mAb1 (high immunogenicity) or active mAb4 (low immunogenicity), the levels of these inflammatory cytokines were lower than when the fragments were tested in a background of media only. In particular, the fragments of all three HTPs that were spiked into active mAb1 showed the lowest secretion of cytokines of all backgrounds tested (media, mAb1, mAb4), even though mAb1 has the highest rate of clinical immunogenicity. This suggests that the immunogenic potential of the inactivated fragments is not affected by the rate of clinical immunogenicity of the mAb that was used to make the IPFs or the mAb that the IPFs are being spiked into. It is important to note that in all cases cytokine levels induced by all samples were much lower than those induced by the positive controls, LPS (Figure 7a) and PHA (Figure 7b). Therefore, cleaning-induced fragments did not increase the risk of immunogenicity of HTP administered to patients at levels relevant to multiproduct manufacturing.
The results indicate that IPFs did not elicit a significant immune-activation response at the concentrations evaluated (10, 100, 200, 650, and 1000 µg/mL). Thus, the AEL of the fragments may be as high as 1000 µg/mL. That level of exposure is about two orders of magnitude greater than the AEL of most therapeutic proteins. It is also consistent with the AEL for inactive therapeutic proteins obtained from in vivo data (1, 2). Further, mAb1B was known to be highly immunogenic and therefore represented a scenario with high immunogenicity risk from a patient-safety perspective. Thus, the results of this study may be more broadly applicable to inactive therapeutic-protein fragments in general.
Discussion
IPFs that form during cleaning and steam sanitization are intrinsic to manufacturing processes for therapeutic proteins. Inactive fragments can carry over into the subsequently manufactured drug product. The impact of such fragments on the induction of early- and late-phase immune responses in human PBMCs was evaluated. Whereas the early-phase response is indicative of the initial immune response, the late-phase response is indicative of a longer-term, T-cell–mediated response, similar to that evaluated using ex vivo T-cell assays (27).
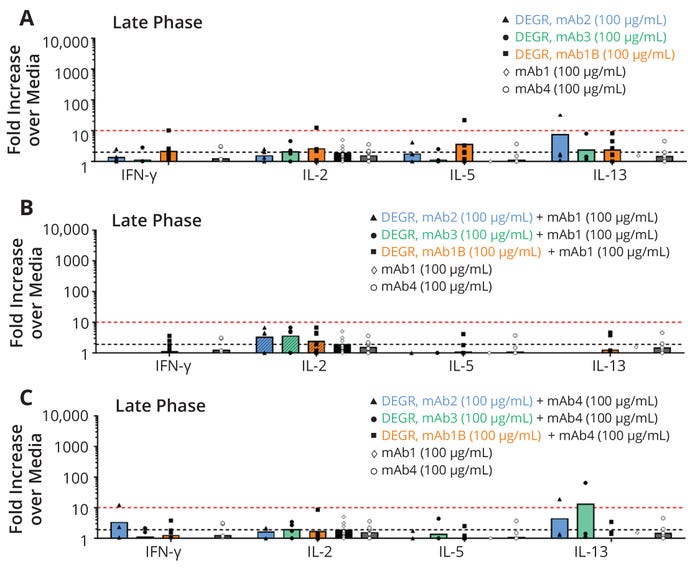
Figure 6: Immunogenicity risk assessment measured by multiplex cytokine release from human peripheral blood mononuclear cells (PBMCs) at seven days to evaluate the impact of degradants (DEGR) from monoclonal antibodies (mAbs) with different rates of clinical immunogenicity (see “Sample Descriptions” at the bottom of this page); the y-axis plots fold increases over values from test media for each cytokine on the x-axis. The different shapes denote individual donor data, and the bars denote the average of all responding donors. The black dotted line represents the assay response threshold (≥2.0×), and the red dotted line is the biological threshold (≥10×). The number of donors for each set of PBMCs ranges from four to 12.
The cytokine signature that was evaluated as a potential biomarker of the in vitro PBMC early-phase response included interleukin 1 receptor agonist (IL-1ra), IL-1α, IL-1β, IL-6, IL-8, IL-10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, tumor necrosis factor α (TNF-α), and TNF-β. The inactive fragments, when challenged at various concentrations ranging from 10 µg/mL to 1000 µg/mL showed no significant early-phase response, and all cytokine levels were well below the biological threshold. It is noteworthy that at the highest concentration (1000 µg/mL), there was a lack of secretion of proinflammatory cytokines at both the early and late phases. A slight increase of IL-10 above the negative control sample was observed for a few samples at both the early and late phase, but the responses were below the biological threshold. The presence of IL-10 could indicate an immune-suppressive effect that may explain the lack of inflammatory cytokines at high doses by shifting the cytokine balance (28–30). Alternatively, a high dose of protein could induce tolerance, which would be accompanied by induction of tolerogenic antigen-presenting cells (APCs) and secretion of IL-10 and TGF-β (28–30). Recent studies also suggest that the fragment crystallizable (Fc) regions of antibody-based therapeutic proteins contain T-regulatory epitopes that can change the balance of T effector cells toward a less inflammatory and more immune-suppressive profile (31). Note that in vitro assessments such as ours can help establish a threshold for IPFs but may not correlate to clinical observations.
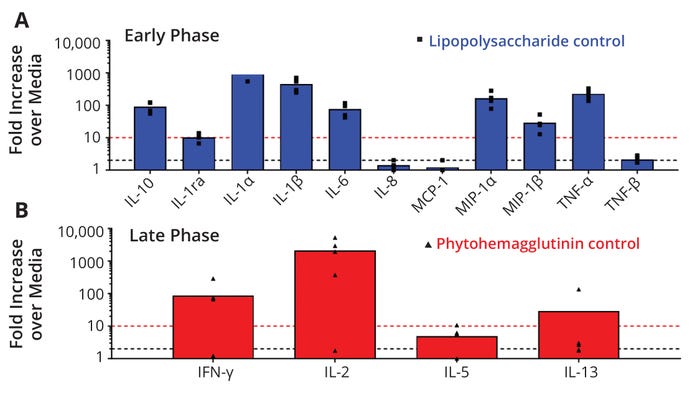
Figure 7: Immunogenicity risk assessment measured by multiplex cytokine release from human peripheral blood mononuclear cells (PBMCs) to evaluate the impact of (a) a lipopolysaccharide control for early phase (20 hours) immune responses to monoclonal antibody degradants (mAb DEGRs) and (b) a phytohemagglutinin control for late-phase (seven days) responses. The y-axis plots fold increases over values from test media for each cytokine on the x-axis. The different shapes denote individual donor data, and the bars denote the average of all responding donors. The black dotted line represents the assay response threshold (≥2.0×), and the red dotted line is the biological threshold (≥10×). The number of donors for each set of PBMCs ranges from three to four.
It is also worth noting that some of the proinflammatory cytokines that were assessed as part of the early-phase cytokine signature (e.g., IL-1α, TNF-α, IL-6, and IL-8) are implicated in the underlying mechanism for immunogenicity-related adverse events following clinical administration (18, 19, 32, 33). Capturing the nature of adverse events in the clinic and monitoring the amount of IPFs in different lots of a commercial product may be useful for developing any associations between presence of such fragments and in vivo inflammation.
In summary, the IPFs of three immunogenic therapeutic proteins tested in media (negative control) and in the presence of active proteins induced no biologically relevant cytokine responses in PBMCs at early or late immune phases. In a few cases, inactivated fragments induced minor responses that were above the assay threshold (≥2×); however, these responses were below the biological threshold (≥10×) and therefore unlikely to result in an early inflammatory response that could propagate immunogenicity to therapeutic proteins in patients (25). Those minor responses do, however, demonstrate the sensitivity of in vitro cell-based assays to detect quality attributes (including inactivated fragments) and are therefore relevant to drug-product development and to mitigating risks from residuals and quality attributes. Immunogenicity of the IPFs appears to affect those minor responses. Inactive fragments from proteins with higher rates of clinical immunogenicity (mAb1B) induced a slightly higher immune response than those from proteins with lower rates of clinical immunogenicity (mAb2 and mAb3); however, the overall response was always below the biological threshold. Also, the presence of active protein appears to play a role but has the opposite effect. In the presence of active protein, low-level responses to the inactive fragments were further diminished, regardless of the immunogenicity of the active protein. This indicates that the presence of inactive protein fragments in active protein formulations at levels relevant to multiproduct manufacturing does not increase the risk of immunogenicity or safety to patients.
Conclusion
Biopharmaceutical cleaning and sanitization cycles are designed to expose product-contact equipment to high pH and temperature for several minutes. Under those conditions, therapeutic proteins and other biologicals degrade into pharmacologically inactive fragments. That phenomenon has important implications for biopharmaceutical cleaning validation. The AEL, and thus the AL for active therapeutic proteins, often is below the capability of most cleaning processes and the LoQs of commonly used analytical methods. Consequently, the AL for active therapeutic proteins usually is not readily achievable. However, the AEL, and thus the AL, for IPFs is typically about two orders of magnitude greater than that of an active protein. Consequently, the AL for IPFs is generally readily achievable.
The higher AL for IPFs can be leveraged to simplify and streamline other aspects of cleaning validation. A higher AL generally results in higher surface and rinse recoveries and fewer cleaning validation failures, which in turn reduces the time and resources required to develop cleaning processes and introduce new products.
The AEL of IPFs was assessed by exposing them in vitro to human PBMCs and subsequently measuring the immune-activation response with human-immune-cell–based assays for cytokines. Immune-cell activation by the IPFs was evaluated in the presence and absence of active therapeutic protein. In the context of operations in a multiproduct facility, the IPFs represent fragments of the API that was previously manufactured in the equipment; the active therapeutic protein represents the API in the subsequently manufactured product.
The results indicate that the AEL of IPFs may be as high as 1000 µg/mL. That level of exposure is about two orders of magnitude greater than the AEL of most active therapeutic proteins. Further, one of the proteins evaluated in this study, mAb1B, was known to be highly immunogenic and thus represented a high-risk scenario from a patient-safety perspective. Thus, the results of this study may be more broadly applicable to IPFs in general.
Acknowledgments
We are grateful to Ram Kouda and Theresa Elsholz for their help.
Declarations
Funding: All experiments were conducted and supported by Amgen.
Conflicts of Interest: Some of the authors are current or previous employees, shareholders, or both employees and shareholders of Amgen, where the work was conducted.
Data Availability: All data needed to evaluate the conclusions in the paper are present in the paper. Upon request and subject to review and legal requirements, Amgen may provide the data that support the findings of this study.

SAMPLE DESCRIPTIONS
mAb1 has a high rate of clinical immunogenicity (>>10%).
Degradants of mAb1 were exposed to both cleaning and sanitization conditions.
mAb1B is the same antibody as mAb1 but comes from a different source. It also exhibits a high clinical immunogenicity rate (>>10%).
Degradants of mAb1B were exposed to both cleaning and sanitization conditions.
mAb2 has an extremely low rate of clinical immunogenicity (<1%).
Degradants of mAb2 were exposed to both cleaning and sanitization conditions.
mAb3 has a low clinical-immunogenicity rate (<7%).
Degradants of mAb3 were exposed to both cleaning and sanitization conditions.
mAb4 shows an extremely low rate of clinical immunogenicity (<1%).
Degradants of mAb4 were exposed to both cleaning and sanitization conditions.
Lipopolysaccharide (LPS) served as a positive control for early-phase immune responses (20 hours after activation).
Phytohemagglutinin (PHA) served as a positive control for late-phase responses (seven days after activation).

References
1 Sharnez R, et al. Cleaning Validation of Multiproduct Equipment — Acceptance Limits for Inactivated Product Based on Gelatin as a Reference Impurity. J. Valid. Technol. 19(1) 2013; https://www.researchgate.net/publication/313361609_Biopharmaceutical_Cleaning_Validation_Acceptance_Limits_for_Inactivated_Product_Based_on_Gelatin_as_a_Reference_Impurity.
2 Sharnez R, et al. Cleaning Validation Acceptance Limits for Biological Process Residues, Part I: Acceptable Exposure of Degraded Proteins Based on Reference Immunogens. BioProcess Int. 21(5) 2023: 26–31, 43; https://www.bioprocessintl.com/validation/cleaning-validation-acceptance-limits-for-biological-process-residues-part-1-acceptable-exposure-of-degraded-proteins-based-on-reference-immunogens.
3 Guideline on Setting Health Based Exposure Limits for Use in Risk Identification in the Manufacture of Different Medicinal Products in Shared Facilities. European Medicines Agency: Amsterdam, Netherlands, 20 November 2014; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-setting-health-based-exposure-limits-use-risk-identification-manufacture-different-medicinal-products-shared-facilities_en.pdf.
4 Guide to Inspections of Validation of Cleaning Processes. US Food and Drug Administration: Rockville, MD, 1993; https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/validation-cleaning-processes-793.
5 Cleaning Validation Guide (GUI-0028). Health Canada: Ottawa, Canada, 2008; https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/validation/cleaning-validation-guidelines-guide-0028/document.html.
6 Sharnez R, To A. Strategies for Setting Rational MAC-Based Limits, Part III: Leveraging Characterization and Toxicological Data. J. Valid. Technol. 17(3) 2011: 24–28; https://www.researchgate.net/publication/313367321_Strategies_for_Setting_Rational_MAC-based_Limits_-_Part_III_Leveraging_Toxicology_and_Cleanability_Data.
7 Sharnez R, et al. Biopharmaceutical Cleaning Validation: Leveraging Acceptable Exposure of Host Cell Protein To Set Acceptance Limits for Inactivated Product. J. Valid. Technol. 18(3) 2012: 38–44; https://www.researchgate.net/publication/303998930_Biopharmaceutical_Cleaning_Validation_Leveraging_Acceptable_Exposure_of_Host-_Cell_Protein_to_Set_Acceptance_Limits_for_Inactivated_Product.
8 Sharnez R, To A. Multiproduct Cleaning Validation: Acceptance Limits for the Carryover of Inactivated API, Part I: The Comparable Quality Approach. J. Valid. Technol. 17(4) 2011: 32–36.
9 Sharnez R, et al. Cleaning Validation of Multiproduct Equipment — Acceptance Limits for Inactivated Product. J. Valid. Technol. 18(2) 2012: 17.
10 Sharnez R. Methodology for Assessing Product Inactivation During Cleaning, Part I: Experimental Approach and Analytical Methods. J. Valid. Technol. 18(4) 2012: 42–45; https://www.researchgate.net/publication/274083889_Methodology_for_Assessing_Product_Inactivation_during_Cleaning_-_Part_I_Experimental_Approach_and_Analytical_Methods.
11 Kendrick K, Canhoto A, Kreuze M. Analysis of Degradation Properties of Biopharmaceutical Active Ingredients As Caused by Various Process Cleaning Agents and Temperature. J. Valid. Technol. 15(3) 2009: 69.
12 Sharnez R. Unpublished Results.
13 Kouda R, Tabassum S, Dolan D. Degradation of Biopharmaceuticals During Cleaning Processes: Comparing Two Different Analytical Methods for Assessment with Bispecific Antibodies. BioProcess Int. 21(6) 2023: 28–38; https://www.bioprocessintl.com/facility-design-engineering/degradation-of-biopharmaceuticals-during-cleaning-processes-comparing-two-different-analytical-methods-for-assessment-with-bispecific-antibodies.
14 Sharnez R. Chapter 8: Importance and Processes of Cleaning. Block’s Sterilization, Disinfection, and Preservation. McDonnell G, Hansen J, eds. Lippincott Williams & Wilkins: New York, NY, 2019.
15 Holley CK, et al. An In Vitro Assessment of Immunostimulatory Responses to Ten Model Innate Immune Response Modulating Impurities (IIRMIs) and Peptide Drug Product, Teriparatide. Molecules 26(24) 2021: 7461; https://doi.org/10.3390/molecules26247461.
16 Haile LA, et al. Detection of Innate Immune Response Modulating Impurities in Therapeutic Proteins. PLoS One 10(4) 2015: e0125078; https://doi.org/10.1038/nri3818.
17 Hanly W, et al. Information Resources for Adjuvants and Antibody Production: Comparisons and Alternative Technologies, 1990–1997. Smith CP, ed. US Department of Agriculture, Animal Welfare Information Center: Washington, DC, 1997.
18 Roche PA, Furuta K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 15(4) 2015: 203–216; https://doi.org/10.1038/nri3818.
19 Jamjian MC, McNicholl IR. Enfuvirtide: First Fusion Inhibitor for Treatment of HIV Infection. Amer. J. Health Sys. Pharm. 61(12) 2004: 1242–1247; https://doi.org/10.1093/ajhp/61.12.1242.
20 Joubert MK, et al. Highly Aggregated Antibody Therapeutics Can Enhance the In Vitro Innate and Late-Stage T-Cell Immune Responses. J. Biol. Chem. 287(30) 2012: 25266–25279; https://doi.org/10.1002/jps.24379.
21 Telikepalli S, et al. Physical Characterization and In Vitro Biological Impact of Highly Aggregated Antibodies Separated into Size-Enriched Populations by Fluorescence-Activated Cell Sorting. J. Pharm. Sci. 104(5) 2015: 1575–1591; https://doi.org/10.1002/jps.24379.
22 Joubert MK, et al. Use of In Vitro Assays To Assess Immunogenicity Risk of Antibody-Based Biotherapeutics. PLoS One 11(8) 2016: e0159328; https://doi.org/10.1371/journal.pone.0159328.
23 Jawa V, et al. Evaluating Immunogenicity Risk Due to Host Cell Protein Impurities in Antibody-Based Biotherapeutics. AAPS J. 18(6) 2016: 1439–1452; https://doi.org/10.1208/s12248-016-9948-4.
24 Joh NH, et al. Silicone Oil Particles in Prefilled Syringes with Human Monoclonal Antibody, Representative of Real-World Drug Products, Did Not Increase Immunogenicity in In Vivo and In Vitro Model Systems. J. Pharm. Sci. 109(1) 2020: 845–853; https://doi.org/10.1016/j.xphs.2019.09.026.
25 Cohen JR, et al. A High Threshold of Biotherapeutic Aggregate Numbers Is Needed To Induce an Immunogenic Response In Vitro, In Vivo, and in the Clinic. Pharm. Res. 41(4) 2024: 651–672; https://doi.org/10.1007/s11095-024-03678-2.
26 Swanson MD, et al. Immunogenicity Risk Assessment of Spontaneously Occurring Therapeutic Monoclonal Antibody Aggregates. Front. Immunol. 13, 2022: e915412; https://doi.org/10.3389/fimmu.2022.915412.
27 Jaber A, Baker M. Assessment of the Immunogenicity of Different Interferon Beta-1a Formulations Using Ex Vivo T-Cell Assays. J. Pharm. Biomed. Anal. 43(4) 2007: 1256–1261; https://doi.org/10.1016/j.jpba.2006.10.023.
28 Mosmann TR, Moore KW. The Role of IL-10 in Crossregulation of TH1 and TH2 Responses. Immunol. Today 12(3) 1991: A49–A53; https://doi.org/10.1016/S0167-5699(05)80015-5.
29 Schülke S. Induction of Interleukin-10 Producing Dendritic Cells as a Tool To Suppress Allergen-Specific T Helper Responses. Front. Immunol. 9, 2018: 455; https://doi.org/10.3389/fimmu.2018.00455.
30 Rasquinha MT, et al. IL-10 as a Th2 Cytokine: Differences Between Mice and Humans. J. Immunol. 207(9) 2021: 2205–2215; https://doi.org/10.4049/jimmunol.2100565.
31 De Groot AS, McMurry J, Moise L. Prediction of Immunogenicity: In Silico Paradigms, Ex Vivo and In Vivo Correlates. Curr. Opin. Pharmacol. 8(5) 20008: 620–626; https://doi.org/10.1016/j.coph.2008.08.002.
32 Vultaggio A, Maggi E, Matucci A. Immediate Adverse Reactions to Biologicals: From Pathogenic Mechanisms to Prophylactic Management. Curr. Opin. Allerg. Clin. Immunol. 11(3) 2011: 262–268; https://doi.org/10.1097/aci.0b013e3283464bcd.
33 Descotes J, Vial T. Flu-Like Syndrome and Cytokines. Cytokines in Human Health: Immunotoxicology, Pathology, and Therapeutic Applications. House RV, Descotes J, eds. Humana: Totowa, NJ, 2007; 193–204.
Previously in Amgen’s clinical immunology group, Vibha Jawa ([email protected]) is executive director for biotherapeutics bioanalysis in nonclinical disposition and bioanalysis at Bristol Myers Squibb, and Jonathan Herskovitz is a researcher in the University of Nebraska Medical Center’s Department of Pharmacology and Experimental Neuroscience. Formerly with Amgen’s process development (PD) group, Rizwan Sharnez ([email protected]) is founder and president of Cleaning Validation Solutions; Michelle Monk is a senior program manager, and Angela Vera (née To) is a laboratory engineer III, both at Hyde Engineering + Consulting. Joseph R. Cohen is a senior principal scientist and team lead, and Marisa K. Joubert is scientific director and group leader in Amgen PD.
You May Also Like






