Viral Clearance in Antibody Purification Using Tentacle Ion ExchangersViral Clearance in Antibody Purification Using Tentacle Ion Exchangers

A researcher at MilliporeSigma, the life science business of Merck KGaA, Darmstadt, Germany, investigates the ability of tentacle ion-exchange media to remove viruses effectively in monoclonal antibody purification processes.
Manufacturers strive toward cost-effective purification of target molecules and a high level of confidence that their biologics are safe and not compromised by the presence of endogenous retrovirus-like particles or adventitious viruses (1). Reliable reduction of viral particles throughout downstream purification processes must be ensured through different techniques such as chemical treatment, filtration, and chromatography. Common monoclonal antibody (MAb) purification schemes use both cation- and anion-exchange chromatography steps (CEX, AEX). Although CEX (to remove product- and process-related impurities) is not generally considered robust enough for virus reduction, in some conditions it has made a significant contribution to virus removal. By contrast, AEX (a polishing step in MAb purification) is generally considered as a robust step for virus reduction.
Tentacle ion exchangers are chromatography media comprising porous hydrophilic polymeric base beads with grafted functional polymers, which can be either AEX (e.g., trialkylammonium, dialkylammonium) or CEX groups (e.g., sulfonate, carboxyl) on particle surfaces. Those surface polymers (tentacles) interact with charged groups or patches on biomolecule surfaces and efficiently bind impurities or target molecules of interest under appropriate operating conditions. Tentacle ion exchangers have been shown to successfully remove viruses in different MAb purification applications.
This article provides a comprehensive review of previously published applications of tentacle ion-exchange media for virus removal in the purification of MAbs. Different types of tentacle chromatography media (e.g., CEX, AEX), as well as various modes of operation (e.g., bind and elute, flowthrough, weak partitioning) will be discussed.
Tentacle Cation Exchangers
Downstream MAb purification schemes link several unit operations that offer multiple opportunities for virus removal using different mechanisms. Some techniques rely on chemical sensitivity of viruses, others on virus charge or size.
In bind–elute mode, viruses can be separated from a target protein using linear- or step-elution processes, taking advantage of different binding strengths of virus and target proteins to the resin used. Such different binding strengths result in different salt concentrations required for elution of bound moieties and enable separation of viruses from bound protein under appropriate elution conditions. In antibody purification, bind–elute mode often is used in CEX intermediate or polishing chromatography.
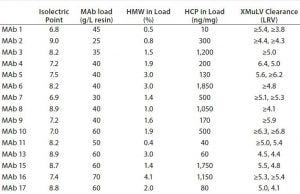
Table 1: Characteristics of molecules and achieved xMuLV (log-reduction values (LRV) clearance with Fractogel EMD SO-3 media at pH 5.0 (only data corresponding to >4 LRV shown) (2)
Connell-Crowley et al. investigated several CEX resins with regard to their ability to separate viruses from antibodies in bind–elute mode (2). The authors demonstrated that Fractogel EMD SO3 – (M) resin (a methacrylate-based tentacle ion exchanger) effectively and reproducibly removed xenotropic murine leukemia virus (xMuLV), a retrovirus. The resin also effectively removed >5 logs of pseudorabies virus (PRV) in all feed streams tested and >5 logs of Reo-3 virus in two of five feed streams. They observed xMuLV clearance of >4 log reduction values (LRV) at pH 5.0 for 15 of 17 MAb feeds (88%) with different characteristics, impurity levels, and at different loadings (Table 1). In all experiments, product yields of ≥90% were obtained.
Those results demonstrate that the Fractogel EMD SO3 – (M) medium shows very robust xMuLV clearance regardless of impurity profile, isoelectric point (pI) of MAb, and resin load. However, success in virus clearance depends on run conditions. For xMuLV and Reo-3, clearance was reduced when the pH of the buffer was increased from 5.0 to 6.0. The data suggested weaker electrostatic interactions between the virus and the resin at higher pH, resulting in coelution of virus and MAb product and consequently reduced levels of viral clearance.
A comparison between different Fractogel cation exchangers showed stronger binding of xMuLV to Fractogel EMD SO3– (M) (a strong CEX resin) than to Fractogel EMD COO (M) medium (a weak cation exchanger). The higher binding strength of the virus to strong CEX resin results in higher elution conductivities for the virus and a more efficient separation of xMuLV from the MAb. The researchers made a similar observation when they compared xMuLV elution profiles from the methacrylate-based tentacle CEX resin Fractogel EMD SO3– (M) with the agarose-based SP Sepharose Fast Flow cation exchanger (from GE Healthcare Life Sciences). Fractogel EMD SO3– (M) showed higher binding strength and elution of xMuLV with higher salt concentration, implying more efficient separation and improved viral clearance. The binding capacity of Fractogel EMD SO3– (M) resin is significantly higher than that of SP Sepharose Fast Flow cation exchanger (3–5). The elution window of xMuLV on Fractogel EMD SO3– (M) resin at pH 5.0 was observed to be 350–800 mM NaCl. By contrast, all MAbs listed in Table 1 eluted at significantly lower sodium chloride levels than xMuLV under those conditions. Consequently, effective separation of virus from a MAb on Fractogel EMD SO3– (M) resin in bind–elute mode can be achieved reliably when a target protein is eluted at a lower ionic strength than the virus.
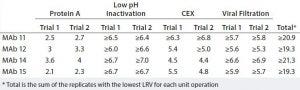
Table 2: XMuLV clearance (LRV) achieved by purification processes using two chromatographic steps (protein A and CEX chromatography) (2)
Because the Fractogel EMD SO3 – (M) resin provides robust, effective clearance of xMuLV, it is possible to use CEX resins in a purification process with only two chromatographic steps. Table 2 shows four examples of MAb processes with a two-column chromatography scheme (protein A followed by CEX in bind–elute mode), including low-pH inactivation and viral filtration steps. That scheme enabled an overall retrovirus clearance of >19 logs and met all other product quality targets.
Eshmuno HCX and Eshmuno CPX resins represent a new generation of polyvinyl-ether–based tentacle ion exchange media. Eshmuno HCX is a multimode tentacle CEX resin that can bind proteins at elevated salt concentrations and offers an alternative to protein A for direct capture of MAbs. Virus reduction across Eshmuno HCX resin was evaluated using a clarified cell harvest containing 1.5 g/L purified MAb at pH 6.0 and 11.3 mS/cm spiked with minute virus of mice (MVM) or xMuLV (3). Columns were loaded to 19 g/L, washed, and the bound product eluted with a step gradient of 20 mM phosphate buffer containing 1 M NaCl, pH 7.0. Fractions containing protein were assayed for virus titer. No MVM clearance was observed on Eshmuno HCX resin with LRV <1, but levels of MuLV reduction were consistently higher at about 2 logs (run 1: LRV = 2.5; run 2: LRV = 2.0).
Eshmuno CPX media is a strong cation-exchange resin with a similar tentacle structure as Fractogel EMD SO3– (M) media and is designed to reduce levels of MAb aggregates in bind–elute mode. Virus reduction across Eshmuno CPX resin was evaluated using a MAb post-protein A pool (11.5 g/L, 8.0 mS/cm, pH 4.7) spiked with MVM or xMuLV. Eshmuno CPX media columns were loaded to 40 g/L, washed, and the bound product eluted with a step gradient of 50 mM sodium acetate buffer containing 1 M NaCl, pH 4.7. Analysis of protein-containing fractions for virus infectivity showed no MVM reduction, similar to results shown previously for Fractogel EMD SO3 – (M) and Eshmuno HCX media. However, unlike Eshmuno HCX resin, no infectious retrovirus was detected in the elution pool of Eshmuno CPX resin, resulting in LRV ≥ 4.1. Under conditions tested, Eshmuno CPX resin demonstrated effective retrovirus clearance.
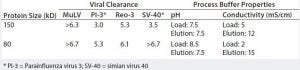
Table 3: Viral clearance validation on Fractogel EMD TMAE (M) resin (6)
Tentacle Anion Exchangers
In 1998, Walter et al. published a summary of virus validation studies that used various ion-exchange resins and several protein feed streams (6). In general, AEX chromatography effectively removed a broad range of viruses under different operating conditions. The authors highlighted that viral clearance was more consistent for Fractogel EMD TMAE (M) resin than for other AEX media throughout various virus types (Table 3). The consistency of the results was attributed to the tentacle-type surface structure of Fractogel EMD TMAE (M) media, which offers multipoint attachments to supramolecular structures such as viruses.
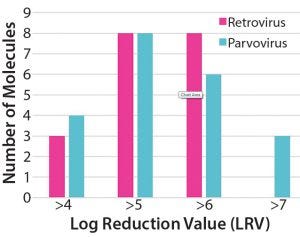
Figure 1: Pfizer´s historical virus clearance data for retrovirus and parvovirus using Fractogel EMD TMAE Hicap (M) resin (7)
By contrast with the operation of anion exchangers in flow-through mode, — which is considered a reliable step for virus removal (9) — AEX chromatography in weak-partitioning mode is characterized by stronger binding of target protein and impurities to the resin. Operational space is defined in terms of the distribution coefficient Kp of the target protein. Kp is equal to the ratio of concentration of adsorbed solute and its concentration in solution under equilibrium conditions. According to Iskra et al., weak partitioning chromatography at Pfizer is typically performed at 1≤ Kp ≤3 (7). Historical data from Pfizer show that AEX chromatography using Fractogel EMD TMAE Hicap (M) resin under weak-partitioning conditions is robust with regard to retrovirus and parvovirus clearance (Figure 1) (7).
The study showed >4 LRV clearance achieved for both retro- and parvovirus for over 20 MAbs across a range of process conditions, including protein load (50–250 g/ L resin), pH (7.0–8.3) and chloride concentration (10–90 mM). These clearance levels were observed with feeds containing a range of impurity profiles: high–molecular-weight (HMW) levels of 1–14%, HCP levels of 500–40,000 ng/mg, and DNA levels <1,500 ng/mg. The fact that high virus clearance was achieved for such a large number of molecules despite the heterogeneity of operating conditions and feed composition highlights the robustness of AEX chromatographic operation for virus reduction (7).
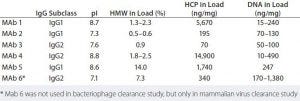
Table 4: Characteristics of molecules used in viral clearance studies with Fractogel EMD TMAE Hicap (M) resin under weak partitioning conditions (7)
To put a historical view into a systematic perspective, researchers performed extensive studies to investigate virus clearance under weak partitioning conditions using bacteriophages Phi X174 (pI of 6.7–7.0) and PP7 (pI of 4.3–4.9) as surrogates for mammalian viruses. Five humanized IgG1 or IgG2 antibody feed streams with various pI values and impurity levels were used (Table 4). All feed streams had undergone protein A capture chromatography before the Fractogel EMD TMAE Hicap (M) chromatography step.
The full-factorial design of experiment (DoE) assessment included the effect of variations in feed stream type, partitioning coefficient Kp, protein load, and column-bed height. Product Kp values were obtained by HTS methods (10). Target Kp values were adjusted accordingly through pH and counterion concentration. Under Pfizer´s typical operating conditions, which are represented by Kp = 1, robust phage clearance was obtained. More specifically, clearance of PP7 with LRV of 5.6–8.58 was obtained for 21 of 22 cases, indicating extremely robust PP7 phage removal. Phi X174 was efficiently removed for MAb 1, 4, and 5 in all experimental scenarios with LRV between 4.38 and 6.62. However, insufficient Phi X174 clearance for MAb 2 and MAb 3 highlighted characteristics of the feed stream and indicated poor removal of neutral viruses under weak partitioning conditions.
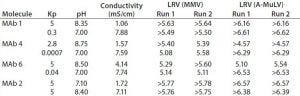
Table 5: Summary of mammalian viral clearance results obtained with Fractogel EMD TMAE Hicap(M) resin (7).
In a follow-up clearance study, MVM and Abelson murine leukemia virus (A-MuLV) were model viruses. The study spanned operating conditions relevant to commercial manufacturing with bracketing Kp values representing flow-through (Kp << 1) and weak partitioning (Kp = 5) (Table 5).
Overall, those studies demonstrate that mammalian virus clearance using Fractogel EMD TMAE Hicap (M) media in MAb purification is robust and effective under weak partitioning conditions and for flow-through operation (Kp << 1).
The newer Eshmuno Q media is a strong AEX tentacle resin based on a polyvinyl ether matrix. Like Fractogel EMD TMAE Hicap (M) media, it can be used for polishing purification of MAb process streams in flow-through mode. The study evaluated virus reduction across Eshmuno Q resin in flow-through mode using post–protein A pool of a MAb feed stream (5 g/L, 2.9 mS/cm, and pH 7.5) spiked with either porcine parvovirus (PPV) or xMuLV (3). For PPV, LRV of 6.6 (run 1) and ≥5.7 were obtained. Retrovirus MuLV could be reduced by ≥ 5.0 logs (run 1) and ≥ 4.7 logs (run 2), respectively. The fact that virus reduction of >4.7 logs was observed for both MuLV and PPV demonstrates that Eshmuno Q resin (like Fractogel EMD TMAE Hicap (M) resin) can be considered to be an effective contributor to both parvovirus and retrovirus clearance when operated in flow-through mode.
A Proven Approach
Tentacle CEX and AEX media of Fractogel EMD and Eshmuno resin families have been evaluated extensively for their ability to remove adventitious and endogenous viruses in MAb purification processes. Published studies cover a wide range of experimental conditions and modes of operation (e.g., bind–elute, flow-through, weak partitioning) and evaluate MAb feed streams with different characteristics using a panel of viruses (e.g., MuLV, PRV, MVM, PPV, Reo-3, and bacteriophage). The effectiveness of tentacle AEX resins in flow-through mode for virus removal is well accepted. The results of studies reviewed herein further demonstrate that tentacle CEX resins can make a valuable contribution to the overall virus removal targets of a MAb purification process and should be considered as a component of an overall virus safety strategy.
References 1 ICH Q5A (R1), 1997. Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology; Step 5: Note for Guidance on Quality of Biotechnology Products — Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. Fed. Register 63(185) 24 September 1998: 51074.
2 Connell-Crowley L, et al. Cation Exchange Chromatography Provides Effective Retrovirus Clearance for Antibody Purification Processes. Biotechnol. Bioeng. 109(1) 2012: 157–165. doi: 10.1002/bit.23300.
3 Pabst TM, et al. Binding and Elution Behavior of Proteins on Strong Cation Exchangers. J. Chrom. A 1216(45) 2009: 7950–7956. doi: 10.1016/j.chroma.2009.09.040.
4 Staby A, et al. Comparison of Chromatographic Ion-Exchange Resins: Strong and Weak Cation-Exchange Resins. J. Chrom. A 1118(2) 2006: 168–179.
5 Staby A, et al. Comparison of Chromatographic Ion-Exchange Resins: Strong and Weak Cation-Exchange Resins and Heparin Resins. J. Chrom A 1069(1) 2005: 65–77.
6 Walter JK, Nothelfer F, Werz W. Virus Removal and Inactivation. Validation of Pharmaceutical Manufacturing. ACS Symposium Series, 1998.
7 Iskra T, et al. Development of a Modular Virus Clearance Package for Anion-Exchange Chromatography Operated in Weak-Partitioning Mode. Biotechnol. Prog. 31(3) 2015: 750–57. doi: 10.1002/btpr.2080.
8 Greenhalgh P, Mehta U. Virus Clearance in Your Process from Start to Finish. BioProc. J. 12(4) 2014: 48–52. http://dx.doi.org/10.12665/J124.Greenhalgh.
9 Miesegaes G, Lute S, Bronson K. Analysis of Viral Clearance Unit Operation for Monoclonal Antibodies. Biotechnol. Bioeng. 106(2) 2010: 238–246. doi: 10.1002/bit.22662.
10 Coffman JL, Kramarczyk JF, Kelley BD. High-Throughput Screening of Chromatographic Separations: Method Development and Column Modeling. Biotechnol. Bioeng. 100(4) 2008: 605–618. doi: 10.1002/bit.21904.
Corresponding author Lars H. Peeck is senior marketing manager, process solutions at the life science business of Merck KGaA, Darmstadt, Germany. Estelle Zelter is senior marketing manager, process solutions at Merck KGaA, Darmstadt, Germany. Patricia Greenhalgh is virology manager, process solutions at MilliporeSigma.
MilliporeSigma is the life science business of Merck KGaA, Darmstadt, Germany. Fractogel and Eshmuno are registered trademarks.
You May Also Like






