Biotech Facilities Average a Batch Failure Every 40.6 WeeksBiotech Facilities Average a Batch Failure Every 40.6 Weeks
September 1, 2008
Gathering information about batch failure rates in the biopharmaceutical industry is about as easy as getting politicians to talk about their most embarrassing gaffes and indiscretions. Although it comes as no surprise that batches do fail, some readers may be surprised at how relatively well many organizations appear to be performing. Based on the results of our recently released annual report and survey (1), facilities are experiencing batch failures at an average rate of about one every nine months (40.6 weeks).
The annual study from BioPlan Associates, Inc., provides an ongoing analysis of worldwide biomanufacturing, with data from 434 biopharmaceutical developers and contract manufacturing organizations in 32 countries. In addition, 126 industry suppliers provided supporting data, and eight subject matter experts provided in-depth analysis. This year’s survey also covers issues such as current capacity, future capacity constraints, expansions, use of disposables (with trends and budgets), trends in downstream purification, quality management and control, hiring issues, employment, and training.
A quantitative trend analysis provides details and comparisons of production by biotherapeutic developers and contract manufacturing organizations (CMOs). The report also evaluates trends over time and assesses differences in the world’s major markets (Europe and the United States).

Figure 1: ()
WWW.PHOTOS.COM
Batch Failure Frequency, Causes
In the report, we evaluated the frequency of batch failures in biopharmaceutical manufacturing (Figure 1). Based on weighted responses from 145 biopharmaceutical organizations worldwide, on average a facility experiences batch failure once every 40.6 weeks (9.4 months). A large percentage (nearly 60%) of respondents had experienced their most recent batch failures within 3–12 months. Further, nearly 80% of the respondents said they had seen no failures in the past three months. For nearly 20%, there had been no failures in the past two years. Although a good percentage of the industry (20%) reported recent failures in the past 90 days, most companies appear to be dealing reasonably well with failure rates.
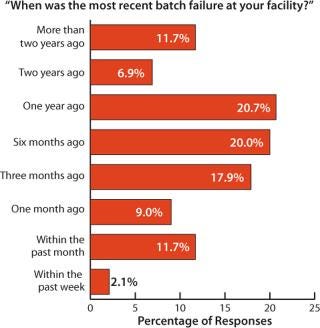
Figure 1: ()
Primary Causes of Batch Failures: We also analyzed the primary causes of batch failures and the percentage of failures in biopharmaceutical manufacturing according to facility capacity (Figure 2). For 1,000-L facilities and larger, the highest rate of failure occurred through contamination (2.3%). The four most significant reasons for batch failure accounted for 80% of all failures. Following contamination, they were operator error, equipment failure, and failure to meet specifications.
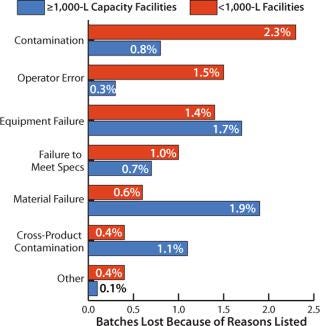
Figure 2: ()
For facilities with less than 1,000 L capacity, the most common cause of batch failures was material failure (1.9%). Product cross-contamination was responsible for 1.1% in these smaller facilities. At such facilities, differences associated with more diverse, smaller-scale product portfolios may have been an important factor in the differences in failure causes.
Clearly, for organizations engaged in investigating and reducing their own failure rates, targeting the largest identified reasons for failure would provide the greatest returns on such efforts.
Total failure rates are similar at 7.6% for >1,000-L scale facilities and 6.6% for <1,000-L scale facilities. However, the distribution of causes appears to vary significantly by scale. According to Beth Junker, PhD (senior director of bioprocess research and development at Merck Research Laboratories in Rahway, NJ), “Although these rates are reasonable in that they are <10%, they are costly owing to high batch raw material and resource costs, often greater than $1–2 million.”
Junker notes that the threefold higher contamination rate experienced by larger facilities — coupled with their significantly higher loss from operator error — suggests areas for improvement in staff training, personnel, and processing at the larger-scale plants. On the other hand, their threefold lower instance of failures due to materials and 2.5-fold lower instance of failures due to cross-product contamination suggests that larger-scale processes do not change as often once they have been implemented. According to Junker, the scale of production may not be a significant factor when comparing failure rates that result from equipment failure or failure to meet specifications. Those relatively similar failure rates in both large and small facilities suggest that the scale of the equipment used does not play a differentiating role.
Batch Failure Remedies
Training appears to be a key factor in reduction of batch failures. Erik Laursen (vice president at CMC Biologics in Mercer Island, WA) says, “Batch failures occur primarily because of training problems. Assuming the equipment’s good, it comes down to the operators’ need to understand basic microbiology — and to getting the basic training in aseptic techniques.” Operating bioreactors and preparing samples aseptically requires staff to be comfortable with aseptic processes. It might seem simple, but this is a subtle concept. Laursen notes, “Every good mother tells her kids,’Go wash your hands.’ But kids’ hands sometimes go unwashed. This translates to biopharmaceutical manufacturing, as well. Staff must be provided with adequate training, not only in aseptic procedures, but also in understanding of the microbiological concepts on which these procedures are based.”
Training in production areas has actually improved significantly over the past three years. For example, in our 2005 survey, over 35% of respondents indicated that increased training in production areas was critical to preventing significant capacity constraints. This year, only 22.7% indicated this factor to be a problem.
Aravind Krishna (director of manufacturing development at Ovation Pharmaceuticals in Lebanon, NJ) concurs: “Batch failures are common to this industry, and they stem from a variety of sources, but especially operator error. A majority could potentially be remedied by providing appropriate training to operators. To address the issue of batch failures, biopharmaceutical manufacturers should be focusing on timing and effectiveness of operator training.”
Improvement Is Possible
Batch failures have a major effect, and can sometimes be devastating, on a biopharmaceutical organization. Reducing failure rates is a priority at most companies; however, achieving 100% quality management in this matter (a zero batch failure rate) is an unrealistic goal. To optimize performance, industry benchmarking makes it possible to assess performance against the industry norm. By identifying trends and trouble spots, quality managers can more effectively focus on primary causes and thus more quickly enact improvements that will translate to lower failure rates overall.
REFERENCES
1.) Langer, E 2008.Fifth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates, Rockville.
You May Also Like






