A Readily Available Source of BSA Consistently Supports Cultivation and Differential Gene ExpressionA Readily Available Source of BSA Consistently Supports Cultivation and Differential Gene Expression
Lyme disease caused by the spirochete Borrelia burgdorferi is the leading vector-borne illness in the United States (1). The natural infectious lifecycle of B. burgdorferi is complex in that it is necessary for the bacteria to colonize both an arthropod vector (the Ixodes scapularis tick, pictured right) and a mammalian host (2). As the bacteria transitions between those two diverse niches, it alters the expression of its major outer surface proteins (Osps) such that expression of those that are no longer necessary for a particular stage of the life cycle are down regulated and those required for colonization or infection are expressed (2).
PRODUCT FOCUS: VACCINES AND LYME DISEASE TREATMENTS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: QA/QC, PRODUCT DEVELOPMENT, PROCESS DEVELOPMENT, AND MANUFACTURING
KEYWORDS: MICROBIOLOGY, BACTERIAL CULTURE, MEDIA OPTIMIZATION, VALIDATION, BOVINE SERUM ALBUMIN
LEVEL: INTERMEDIATE
The best-characterized example of that coordinated response is in production of outer surface protein C (OspC), an Osp required for infection of a mammalian host (2, 3) in response to the conditions encountered in the midgut of a feeding tick or mammalian host tissues (2). Such conditions can be recapitulated in vitro by growing the bacteria to a high cell density (1 × 108 bacteria/mL) at an elevated temperature (36 °C) and reduced pH at 6.8 (4). Characterization of the molecular events responsible for sensing these environmental changes and modulating the bacterial adaptive response, along with the proteins expressed in response to these stimuli, have long been a key focus of Borrelia researchers.
The Barbour–Stoenner–Kelly (BSK) medium developed for in vitro cultivation of B. burgdorferi comes in several formulations (5, 6). Media prepared by individual researchers are typically referred to as BSK-II (5), whereas a commercially available BSK medium is designated BSK-H (6). Although BSK-H and BSK-II typically work equally well for general bacterial cultivation, in vitro B. burgdorferi regulation studies are often complicated by media-dependent differences (7). For OspC regulation studies, BSK-H is typically superior to investigator-prepared BSK-II media (7) despite the fact that the compositions of these two media are roughly equivalent. Although BSK-H is better suited for regulation studies, occasional disruptions in its supplies have led many laboratories to rely on self-prepared BSK-II media.

The black-legged tick (Ixodes scapularis) is the primary vector for Lyme disease. ()
Media selection is also critical when cultivating B. burgdorferi bacteria intended for use in whole-cell–based vaccine preparations. To enhance the efficacy of such a vaccine, bacteria should be cultivated under conditions that provide for maximal expression of potential Borrelial vaccinogens that a mammalian host might encounter during infection (2). Considering this point, BSK-II preparations that are inefficient in induction of OspC by B. burgdorferi would be suboptimal for growing bacteria used in generating an inactivated whole-cell–derived vaccine.
An additional level of complexity is illustrated in a report implicating the bovine serum albumin (BSA) component as a vital factor that can affect growth (8). That report served as a cautionary note suggesting that researchers carefully assess each BSA preparation before its use in BSK preparations. Unfortunately, it is impossible to predict accurately whether a specific BSA will provide adequate growth support and regulation. Thus, whenever a researcher replenishes his or her supply of BSA, thorough testing must be performed to determine the quality of the material used. Here we describe our identification of a BSA that consistently supports growth of B. burgdorferi and differential expression of OspC, alleviating the need for extensive validation.
Material and Methods
Media Preparation: We prepared our media as described by Alan Barbour with the exception that gelatin was omitted (5). For each BSA comparison, either a 1-L or 2-L batch of BSA-free BSK-II was prepared. For 1 L of BSK-II, reagents were combined to a final volume of 800 mL, which then was divided into four 200-mL aliquots. We added 12.5 g of BSA (5% final concentration) to each aliquot (details of each provided in results section). The pH of each medium was then adjusted to 7.5, and deionized water was added to make a final volume of 250 mL. For growth and regulation assessments to be performed at low-pH conditions, we adjusted each medium to pH 6.8 as previously described (4). In each comparison, we included and similarly treated a sample of BSK-H Complete medium from Sigma-Aldrich (www.sigmaaldrich.com) at pH 7.5.
Assessment of B. burgdorferi Growth Support: To characterize each media for its ability to cultivate B. burgdorferi, 40 mL of media (at both pH 7.5 and 6.8) was inoculated with a low-density culture (<107 bacteria/mL) of B. burgdorferi strain 297 (9) to an initial density of 1 × 103 bacteria per mL. Cultures were incubated static at 36 °C with 3% CO2, and the cell densities assessed by enumerating spirochetes using dark-field microscopy at five (pH 7.5) or seven (pH 6.8) days following inoculation. To ensure the accuracy of bacterial density determination, a minimum of ten microscopic fields were counted.
Assessment of Protein Expression By B. burgdorferi: To assess the induction of OspC by B. burgdorferi, we grew bacteria in the pH 6.8 adjusted media to a high density (∼108 bacteria/mL). It is well established that these are optimal in vitro growth conditions for inducing expression of OspC (7). At seven days following inoculation, the bacterial cell density of each culture was determined as described above, after which an aliquot was collected for analysis.
We prepared bacterial lysates and performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis as previously described (10). A volume of whole-cell lysate equivalent to 2 × 107 spirochetes was loaded per gel lane, and proteins were resolved using 12.5% polyacrylamide gels. Our molecular mass standard was the All Blue Precision Plus Protein standards from Bio-Rad Laboratories (www.bio-rad.com). Resolved proteins were stained with Coomassie brilliant blue protein stain.
Made using darkfield microscopy technique, this 400× photomicrograph reveals the presence of the helical-shaped Borrelia burgdorferi bacteria, the pathogen responsible for causing Lyme disease. ()
Results
Testing of Probumin BSA Products for B. burgdorferi Cultivation: A key component in BSK media, BSA is typically derived by one of two general methods: cold-ethanol fractionation (Cohn Fr V) and heat shock (5, 6, 8). Cohn Fr V BSA is usually suitable for growth of B. burgdorferi; however, it is currently in short supply. As an alternative, some Borrelia researchers are using heat-shock–generated BSAs, but variation in each supplier’s heat-shock processes make it difficult to predict what constitutes a BSA suitable for Borrelia cultivation.
To identify a suitable source of BSA for growth of B. burgdorferi, we tested three Probumin products from Millipore Corporation (www.millipore.com) in individual BSK-II preparations: Vaccine Grade (Catalog #84-065), Universal Grade (#81-003), and Fatty Acid Free (#82-002) BSAs. For growth assessment, we inoculated two aliquots of each medium (pH 7.5 and pH 6.8) to a low density with B. burgdorferi strain 297. Control cultures of BSK-H Complete medium also were inoculated. When the bacterial cell densities of the cultures were determined at five (pH 7.5) or seven (pH 6.8) days following inoculation, all the BSK-II preparations performed as well as BSK-H Complete medium (Figure 1A). Although the bacterial densities in BSK-II containing Vaccine Grade BSA were lower (especially at pH 6.8), the difference was negligible (less than one log lower) compared with the other cultures.
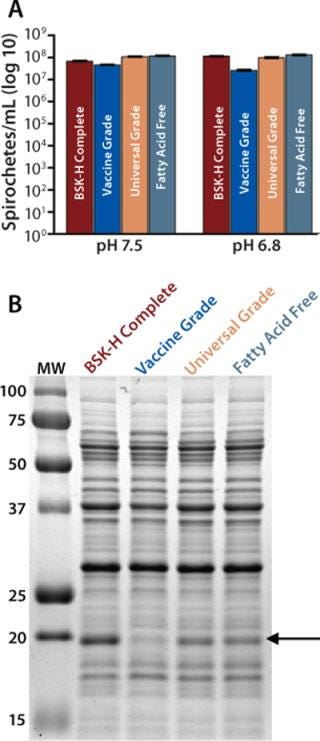
Figure 1: ()
To evaluate the suitability of these BSAs in BSK-II for differential regulation studies, we assessed OspC expression by B. burgdorferi. At seven days following inoculation, bacteria were collected from the pH 6.8 cultures and prepared for analysis by SDS-PAGE (Figure 1B). As expected, we observed significant induction of OspC in spirochetes grown in BSK-H Complete media. Bacteria from the BSK-II cultures prepared with Probumin Universal Grade and Fatty Acid Free BSA also expressed OspC, albeit at a slightly lower level.
That difference in OspC induction observed between BSK-H Complete and BSK-II is consistent with the results of previous studies (7). By contrast, bacteria cultivated in BSK-II containing Vaccine Grade BSA showed no significant OspC expression. Taken together, these results indicate that all the BSAs tested were suitable for general cultivation of B. burgdorferi, but only the Universal Grade and Fatty Acid Free BSAs were suitable for use in gene-expression studies. Although both performed equally, the higher cost of the Fatty Acid Free version makes it less suitable for large-scale applications. So we selected Universal Grade BSA for subsequent analysis.
Assessing Consistency in Universal Grade Preparations: Lot-to-lot variation in manufactured preparations usually make it necessary for researchers to test each lot of a potentially suitable BSA for its capacity to support growth and differential gene expression (8). Therefore, we sought to test three randomly selected lots of Probumin Universal Grade BSA (spanning almost two years of production) to determine its reproducibility. As shown in Figure 2A, the BSK-II we made with each lot of Universal Grade supported growth of B. burgdorferi equally well at either pH. Furthermore, in assessing expression of OspC by the bacteria grown in each preparation, we detected no significant difference among the three lots (Figure 2B). Taken together, these data suggest that Universal Grade BSA is a consistently reliable source that can be considered suitable for cultivation of B. burgdorferi and gene-expression studies.
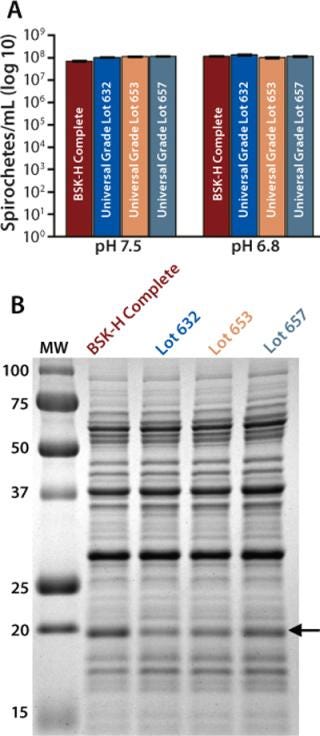
Figure 2: ()
Direct Comparison of Commercially Available BSA Preparations: Numerous sources of BSA products are available, so we next performed a head-to-head comparison of Probumin Universal Grade BSA with others from several commercial sources. We selected four BSAs, arbitrarily labeled A through D, based on either their individual suppliers’ recommendations for use in B. burgdorferi microbiological cultivation or on the recommendation of other researchers.
As seen in our other experiments, spirochete densities in BSK-II prepared using Universal Grade BSA were equal to those measured in BSK-H Complete media (Figure 3A). Only BSA-B performed as well as Universal Grade at both pH conditions. At pH 7.5, the density of bacteria in media prepared with BSA-C was ∼5% of that obtained using the Probumin Universal Grade (>1 log difference). However, by contrast, that same BSK-II preparation supported growth at pH 6.8 equivalent to both BSK-H Complete and Universal Grade BSK-II media. Cell density in the pH 7.5 culture of BSK-II made with BSA-D was >3 logs lower than that of the medium prepared with the Probumin BSA, and at pH 6.8 the BSA-D medium failed to support growth entirely. Performance of BSK-II prepared with BSA-A was also suboptimal at both pH 7.5 and 6.8; cell densities were 2% and 0.7%, respectively, of those observed with the Universal Grade BSA.
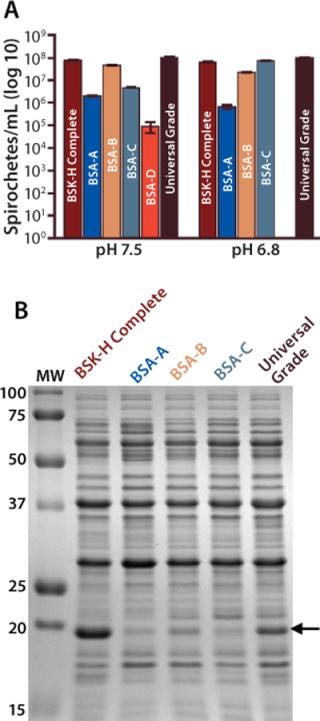
Figure 3: ()
Our SDS-PAGE analysis of bacteria grown in media containing BSAs A, B, and C revealed no significant OspC expression by those harvested from the pH 6.8 cultures (Figure 3B). Because the overall growth performance of media prepared with BSA-A was relatively poor, we found it necessary to grow this culture for an additional three days to obtain enough spirochetes to carry out a comparison. Despite the fact that all cultures we tested reached sufficient cell densities, only bacteria grown in the presence of the Universal Grade BSA showed significant induction of OspC.
The culture grown with BSA-B was the only other one that showed any notable expression of OspC. However, microscopic evaluation of those bacteria revealed that, by contrast with the Universal Grade BSA culture, they were less motile, elongated, and showed evidence of membrane perturbation (data not shown), all of which indicate that bacteria are growing under suboptimal growth conditions (8). Collectively, these data indicate that although several alternate commercial BSA sources will support growth of B. burgdorferi, only BSK-II media made with the Universal Grade BSA is suitable for either differential gene-regulation experiments or cultivation of bacteria in conditions that maximize the expression of potential B. burgdorferi vaccinogens.
Assessing the Impact of γ-Irradiation: During BSA fractionation, infectious agents present in the original bovine material can be carried over into a final product. This is especially important when considering that one potential applications for Probumin Universal Grade BSA is large-scale cultivation of B. burgdorferi for use in a whole-cell–derived vaccine preparation. Unfortunately, the classical practices of sterilizing solid biomaterials using chemical methods (11) or autoclaving cannot be applied to BSA because its exposure to such treatments can cause significant damage to the protein or carryover of residual chemicals that could impair its function.
An alternative means of sterilizing proteinaceous materials is treatment with ionizing γ-radiation (12, 13). This technique involves exposing material to an isotope source such as cobalt or cesium, therefore negating the requirement for exposure to denaturing conditions or chemicals. Whereas sterilization by γ-irradiation has a distinct advantage over the more traditional methods, there is still evidence indicating that treatment of BSA with ionizing radiation leads to disruption of the ordered protein structure as well as degradation, cross-linking, and aggregation of the polypeptide chains (14).
We sought to determine whether treatment of Probumin Universal Grade BSA with γ-irradiation would have any adversely affect its ability to support bacterial growth and regulation. For this comparison, we exposed a sample to ∼30 kGy and left a second sample from the same lot untreated. Both were then used to prepare two batches of BSK-II media. As previously stated, exposure to ionizing radiation can lead to increased polymerization and aggregation of BSA, which could impair the solubility of γ-treated BSA (14). Indeed, we did observe a slight decrease in solubility with the irradiated BSA, but that difference was minimal; the irradiated material required only an extra five minutes to dissolve (data not shown).
Comparison of bacterial densities in media prepared with either the treated or untreated Universal Grade BSA revealed no difference between the two in their ability to support growth of B. burgdorferi at either pH 7.5 or 6.8 (Figure 4A). Likewise, the level of OspC expression by bacteria grown in BSK-II prepared with γ-irradiated BSA was equivalent to that observed in media prepared with untreated BSA (Figure 4B). Taken together, these results indicate that treatment of Universal Grade BSA with ionizing irradiation had no demonstrable impact on its performance.
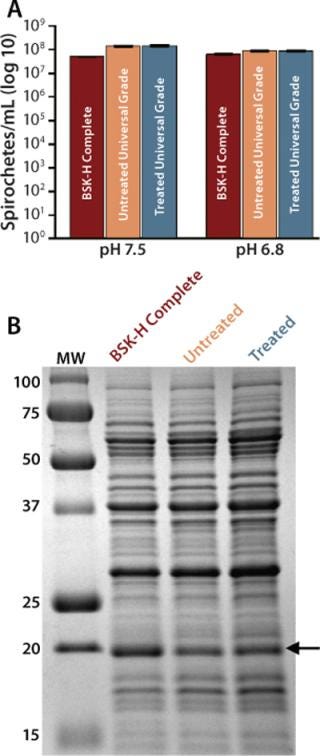
Figure 4: ()
Discussion
To identify a reliable source of BSA, we tested several Probumin products from Millipore for their capacity to support growth of B. burgdorferi. We identified two products, Universal Grade BSA and Fatty Acid Free BSA, that were suitable for use in BSK-II. In addition to positive general cultivation results, we found that media prepared with these BSAs also worked in growing bacteria for differential gene-regulation studies (e.g., induction of OspC). When assessing lot-to-lot consistency in the quality of the Universal Grade BSA over three randomly selected preparations for B. burgdorferi cultivation and OspC induction, we found that all lots (spanning almost two years of production) performed equally. Identification of a BSA that is consistent to this degree should significantly benefit many B. burgdorferi researchers and vaccine developers. Our additional experiments demonstrated that exposure of Universal Grade to γ-irradiation, a treatment that may be desirable if using BSA in the growth of bacteria for a whole-cell–based vaccine, had no demonstrable impact on the ability of BSK-II medium prepared with the treated BSA to grow B. burgdorferi or on differential gene regulation.
The most telling evidence for the superiority of this BSA source came during our comparison of commercially available BSAs. Although BSK-II media prepared with BSA from several sources were capable of supporting growth at both pH conditions tested, only the spirochetes grown with the Universal Grade BSA showed significant expression of OspC. We selected the products to test based on either recommendation of their manufacturers or other Borrelia researchers, but our preliminary comparison revealed no significant commonalities among them.
The fact that both Probumin Universal Grade and Fatty Acid Free BSAs are very low in fatty acid content and performed well suggests that such low concentrations of fatty acid are best for growth and regulation studies. This conclusion is supported by our finding that the other BSAs we tested contain higher levels of fatty acids (data not shown) and subsequently failed to support growth and/or induction of OspC. However, the fact that bacteria grown in BSK-II prepared using BSA-B (which is lipid-enriched) expressed a measurable level of OspC expression disagrees with the hypothesis that BSAs replete in fatty acids are less suitable. However, BSA-B is actually highly purified (Fatty Acid Free) before being supplemented with lipids. Taken together, these data might suggest that for a BSA to be ideally suited to use in B. burgdorferi cultivation, stringent fractionation might be required to remove fatty acids and other contaminating molecules (immunoglobulins and/or antibiotics) bound to it. The impact that the presence of such copurified molecules in growth media might have on B. burgdorferi is unknown, but it has been established that eukaryotic fatty acids can have significant bactericidal activity (15). We now are poised to begin assessing the constituents of the suboptimal BSAs in an attempt to identify factor(s) that might impair growth and/or the expression of OspC. Those molecules could then serve as quality indicators when modifications are made to a fractionation process.
Although it is still not precisely known what constitutes a BSA that can be used for growth and regulation studies in B. burgdorferi, our results have begun to provide a glimpse into the characteristics indicative of a suitable BSA. This is the first such report to do so. We have successfully identified a readily available and consistent source of BSA (the Probumin Universal Grade product from Millipore) that can be used for preparation of BSK growth media. Furthermore, that BSA product was unaffected by treatment with ionizing radiation, making it ideally suited for applications requiring large-scale cultivation of B. burgdorferi, such as in preparation of inactivated whole-cell–derived vaccines. Identification of such a supplement marks a significant advancement for microbiologists working with Borrelia because it negates their need to test each preparation of BSA for its capacity to support growth and affect gene regulation.
REFERENCES
1.) 2007. Lyme disease: United States, 2003–2005. Morb. Mortal. Wkly. Rep. 56:573-576.
2.) Fikrig, E, and S. Narasimhan. 2006. Borrelia burgdorferi: Traveling Incognito?. Microbes Infect. 8:1390-1399.
3.) Tilly, K. 2006. Borrelia burgdorferi OspC Protein Required Exclusively in a Crucial Early Stage of Mammalian Infection. Infect. Immun. 74:3554-3564.
4.) Yang, X. 2000. Interdependence of Environmental Factors Influencing Reciprocal Patterns of Gene Expression in Virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479.
5.) Barbour, AG. 1984. Isolation and Cultivation of Lyme Disease Spirochetes. Yale J. Biol. Med. 57:521-525.
6.) Pollack, RJ, SR Telford, and A. Spielman. 1993. Standardization of Medium for Culturing Lyme Disease Spirochetes. J. Clin. Microbiol. 31:1251-1255.
7.) Yang, X. 2001. Influence of Cultivation Media on Genetic Regulatory Patterns in Borrelia burgdorferi. Infect. Immun. 69:4159-4163.
8.) Callister, SM. 1990. Effects of Bovine Serum Albumin on the Ability of Barbour–Stoenner–Kelly Medium to Detect Borrelia burgdorferi. J. Clin. Microbiol. 28:363-365.
9.) Hughes, CA, CB Kodner, and RC. Johnson. 1992. DNA Analysis of Borrelia burgdorferi NCH-1, the First Northcentral US Human Lyme Disease Isolate. J. Clin. Microbiol. 30:698-703.
10.) Yang, X. 1999. Identification, Characterization, and Expression of Three New Members of the Borrelia burgdorferi Mlp (2.9) Lipoprotein Gene Family. Infect. Immun. 67:6008-6018.
11.) Spire, B. 1984. Inactivation of Lymphadenopathy Associated Virus By Chemical Disinfectants. Lancet 2:899-901.
12.) Plavsic, MZ. 1999. Gamma Irradiation of Bovine Sera. Dev. Biol. Stand. 99:95-109.
13.) Spire, B. 1985. Inactivation of Lymphadenopathy-Associated Virus By Heat, Gamma Rays, and Ultraviolet Light. Lancet 1:188-189.
14.) Gaber, MH. 2005. Effect of Gamma-Irradiation on the Molecular Properties of Bovine Serum Albumin. J. Biosci. Bioeng. 100:203-206.
15.) Thormar, H, H Hilmarsson, and G. Bergsson. 2006. Stable Concentrated Emulsions of the 1-Monoglyceride of Capric Acid (Monocaprin) with Microbicidal Activities Against the Food-Borne Bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 72:522-526.
You May Also Like






