Meeting Increased Demands on Cell-Based Processes By Using Defined Media SupplementsMeeting Increased Demands on Cell-Based Processes By Using Defined Media Supplements
September 1, 2011
Rapidly increasing demand for cell-derived products has placed huge pressures on the biomanufacturing industry’s production capacity requirements. Media development strategies continue to be a primary focus for optimizing output from cell culture systems. Animal cells used in manufacturing protein products have complex nutrient requirements specific for each cell type, clone, and product. Individual nutrient requirements were once addressed by using serum-based media rich in growth factors and supplements, which provided an optimal culture environment for cell growth and productivity (1). However, the use of serum is gradually being phased out in biopharmaceutical manufacture. Seasonal and continental differences in serum composition and batch-to-batch variations lead to inconsistent cell culture performance and product quality. Moreover, because of contamination risk from blood-borne adventitious agents (mycoplasma, viruses, and prions), regulatory agencies strongly discourage the use of animal-derived products in biologics manufacturing.
PRODUCT FOCUS: PRODUCTS OF ANIMAL CELL CULTURE
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: PROCESS DEVELOPMENT, QA/QC, ANALYTICAL LABORATORIES, MANUFACTURING
KEYWORDS: ANTIBODIES, VACCINES, RAW MATERIALS, QBD, ROBUSTNESS
LEVEL: INTERMEDIATE
Those factors have created a demand for development of serum-free (and more recently chemically defined, protein-free) media that deliver optimal cell growth and productivity (2). Because of specific nutrient requirements for different production cell lines, a media formulation must be tailored to help each process achieve optimal growth. To date, it has not been possible to design a single serum-free or chemically defined media formulation that can act as a serum substitute suitable for growing all cell lines. In fact, different clones of the same cell type even require different formulations for optimal growth, and media optimization for each can be extremely time consuming and costly. Biomanufacturers are demanding robust media platform technologies that can be applied across their production portfolios to deliver the required upstream process and productivity output while maintaining product quality.
Here I discuss current media development strategies designed to optimize output from cell culture systems, industry requirements for robust media platforms, and the role of defined media supplements in addressing those. Using experimental examples, I examine the benefits of supplementing commercially available, chemically defined media with our company’s LONG R3 IGF-I, rTransferrin, and rAlbumin ASP-G defined recombinant supplements. I show how they may be applied across the upstream production process, from cryopreservation of mammalian cells to large-scale manufacture of recombinant proteins and monoclonal antibodies (MAbs). In addition, I explore the benefits of defined media supplements for the manufacture of cell-derived vaccines and emerging technologies.
Animal-Free Cryopreservation
Stable storage of production cell lines in master and working cell banks is a fundamental requirement for all biomedical manufacturers. Cryopreservation is widely accepted as the method for long-term storage. To ensure optimal cell recovery, this technique has historically required incorporation of fetal bovine serum (FBS) in cryopreservation media. However, animal-free alternatives must be investigated in response to increasing pressure from regulatory agencies, especially when cryostorage precedes an animal-free biomanufacturing process.
Our group examined an animal-component–free (ACF) human recombinant albumin (rAlbumin) for its ability to replace serum in cryopreservation of Chinese hamster ovary (CHO) cells expressing anti-IL-8 MAbs. Cells were maintained in commercially available, chemically defined, serum-free media (CD-SFM) with GlutaMAX supplement from Invitrogen and Methotrexate (MTX) antimetabolite. Cells were frozen at a density 1×107 in FBS with dimethyl sulfoxide (DMSO) polar solvent (medium 1), rAlbumin with DMSO and CD-SFM (medium 2), or a commercial cryoprotection media (medium 3). After thawing, cells were recovered in CD-SFM and analyzed for viable-cell density (VCD), percent cell viability, and percent midapoptosis using Millipore’s Guava ViaCount assay over three days.
Our results demonstrated that all three media were effective for cryopreservation. Growth of CHO cells was comparable over three days of recovery in CD-SFM, but cells cryopreserved in rAlbumin medium demonstrated enhanced growth compared with the FBS or commercial cryoprotection medium (Figure 1). After three days of growth, although the percentage of viable CHO cells frozen in media containing rAlbumin was comparable to that of cells frozen in FBS or the commercial cryoprotection medium, the number of cells in midapoptosis was reduced for those frozen with rAlbumin (Figure 2).
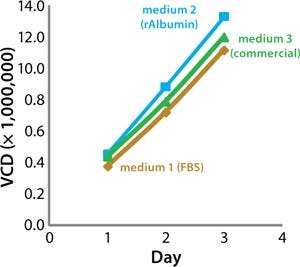
Figure 1:
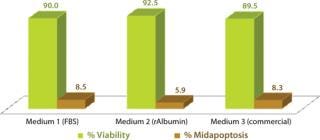
Figure 2: ()
Robust Media Platforms with Defined Supplements
A CD-SFM strategy delivers many benefits for the manufacture of biotherapeutics, not the least of which are regulatory compliance, simplified downstream processing, and consistency of both processes and products (3). All are important drivers in reducing drug development costs for biomanufacturers, which should translate to affordable treatments for patients. Because of the different nutrient requirements for various production cell lines, however, each line requires its own specific media formulation to achieve optimal performance. Optimization can be time consuming, and maintaining culture viability while improving product titers can be challenging. Chemically defined, serum-free conditions often need to be supplemented with a growth factor or bioactive supplement for robustness (4). In addition, protein-free environments can promote nonspecific adsorption of cell-derived products to material surfaces, increased sensitivity of cells to bioreactor shear forces, biophysical stresses such as oxidation, and proteolytic degradation (2,4).
We compared two commercially available, animal-free recombinant growth factors — insulin and our company’s LONG R3 IGF-I supplement — for their ability to stimulate cell growth and productivity in commercially available CD-SFM. Both factors are used in the serum-free manufacture of many biologics across a range of cell lines. Their mitogenic function is often essential for achieving and maintaining the viable cell densities needed for optimal performance under production conditions.
To study the benefit of supplementing CD SFM with a growth factor, we seeded cells at 1×105 cells/mL culture media containing either growth factor in 50-mL TTP tubes and incubated them for 11 days. Cultures were grown in duplicate and then sampled in duplicate for each time point (n = 4). After harvest, we analyzed samples for cell viability and apoptosis with the ViaCount assay, and for productivity determined by affinity HPLC (with a protein A HPLC column) to measure IgG levels.
CHO cell viability was similar in all cultures at day 6; however, toward the end of the culture period, the LONG R3 IGF-I supplement supported higher cell viability (Figure 3, TOP). The number of cells in midapoptosis was also reduced in its presence (compared with both CD-SFM alone and with insulin) in the later stages of the batch culture (Figure 3, CENTER). The observed maintenance of cell viability in cultures treated with LONG R3 IGF-I growth factor also translated to a significant increase in product titer (Figure 3, BOTTOM).
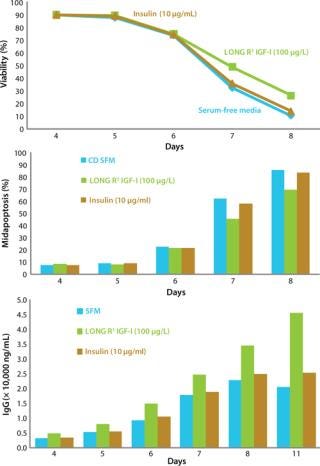
Figure 3: ()
We also examined the rAlbumin ASP-G (rAlbumin) animal-free recombinant albumin in a separate study for its ability to protect CHO cells against oxidative stress. Using hydrogen peroxide (H2O2) treatment as a model for induced apoptosis through oxidative stress, we tested rAlbumin for its potential antioxidant properties. We seeded CHO cells at 1×105 cells/mL in 50-mL TTP tubes and added H2O2 to a final concentration of 0.08 mM±rAlbumin (1 mg/mL). After harvest, we analyzed our samples for midapoptosis and growth with the ViaCount assay and for productivity using affinity HPLC (with a protein A HPLC column) measurement of IgG levels.
Our results demonstrate that CHO cells treated with H2O2 plus rAlbumin displayed reduced midstage apoptosis compared with cells stressed by peroxide treatment alone (Figure 4, TOP). Moreover, we observed growth and productivity equivalent to controls (SFM and rAlbumin) in CHO cells treated with H2O2 plus rAlbumin compared with those treated with H2O2 alone, which displayed reduced cell growth and productivity (Figure 4, CENTER and BOTTOM).
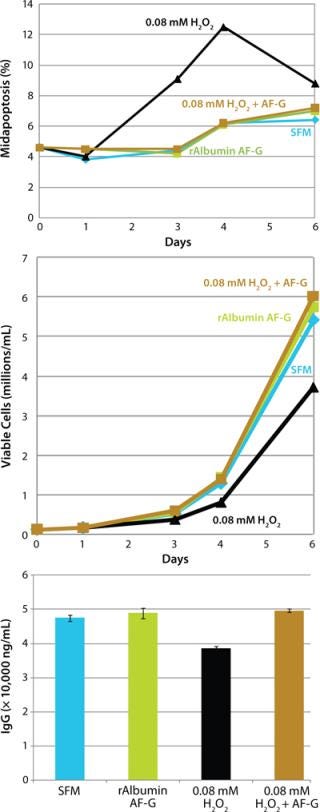
Figure 4: ()
Cell-Based Therapies in an Animal-Free Environment
Mammalian cell culture is increasingly the system of choice for production not only of biological pharmaceutical proteins, but also of emerging therapies such as viral vaccines, stem cells, and tissue therapies. Similar to the production of biological proteins, these product classes have seen a necessary shift away from use of serum-containing media or any animal-derived products in upstream manufacture because of regulatory concerns over media safety risks, variability, and cost (5). Whereas the movement to a serum-free media environment for manufacturing recombinant proteins and antibodies has been relatively rapid for suspension cultures (e.g., CHO cells), it has been a more difficult task for vaccine cell lines and cell-derived therapies.
Traditionally, adherent vaccine cell types such as Vero and MDCK require additional molecules to promote their attachment, growth, or virus production in culture (6). Generating suspension cell lines for manufacturing vaccines for influenza and other applications is a current area of focus. Suspension cells simplify production scale-up by eliminating the need for attachment surfaces as well as detachment and reattachment steps between cultivation (7).
Use of defined protein media supplements in development of ACF media for improved growth and productivity of vaccine-related cell lines has been investigated for both adherent and suspension cell types (8). Our preliminary study tested growth responses to a selection of bioactive proteins in two cell lines (Vero and MDCK) under investigation for commercial production of viral vaccines: insulin-like growth factor (LR3), epidermal growth factor (LEGF), transferrin (rTF), and albumin (rAlb).
To demonstrate how defined supplements allow rapid transition from a serum-containing culture environment to serum free, we maintained Vero and MDCK cells separately in a basal medium of Dulbecco’s modified Eagle’s medium with Ham’s nutrient mixture F12 (DMEM/F12) supplemented with 10% (v/v) FBS and 2 mM GlutaMAX supplement. Cells were harvested, washed with DMEM/F12 basal medium, counted, and then diluted in basal medium with 2 mM GlutaMAX media. A cell suspension containing 4,000 cells was seeded into a 96-well culture plate, to which the supplements to be tested were added either alone or in combination. Cell growth was assessed using Invitrogen’s CyQUANT NF cell proliferation assay kit, with results expressed as relative fluorescence units (RFU).
Our results show that Vero (Figure 5) and MDCK (Figure 6) cells showed increased growth responses over the serum-free control when recombinant supplements were used in combination. When all four supplements were used together, the growth response approached that achieved using 10% FBS. The cell lines we tested had not been previously adapted to these supplements, which demonstrates the possibility for direct substitution from serum-containing media to SFM for vaccine cell culture.
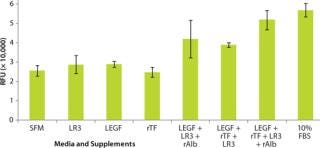
Figure 5: ()
< br clear="all">
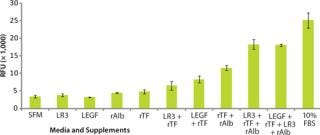
Figure 6: ()
The Importance of High-Quality Components
Animal-free media supplements for large-scale manufacturing are becoming increasingly available to upstream media development scientists. A primary reason for shifting away from the use of serum is its complex and ill-defined nature. Variations among batches of serum can cause phenotypic variations in cell cultures, which in turn affect product quality (2). With implementation of quality by design (QbD) concepts in the biopharmaceutical industry, quality has increasingly become intrinsic at all stages of biomanufacturing (9). Defined as “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management,” QbD is often applied at the product/process development stage (10). If it is to be applied to complete biomanufacturing processes, then it must also involve raw materials such as media components, which are often prone to variability and not in direct control of process development scientists. Defined media supplements designed and manufactured exclusively for cell culture are providing proven growth benefits as well as process and performance consistency (11).
Availability of detailed information about raw-material quality specifications can vary among suppliers and depends on their customer relationships. Lack of product knowledge or protection of product formulation information can make it difficult to call some supplements truly defined. Some suppliers work closely with their customers to ensure that all aspects of product quality meet each biomanufacturer’s process requirements. Specific raw material analysis — including HPLC fingerprinting, nuclear magnetic resonance (NMR) analysis, inductively coupled plasma mass spectrometry (ICP-MS), and liquid chromatography with mass spectrometry (LC-MS) — can be provided to give customers a good understanding of the relationship of raw material variations to manufacturing consistency (12). Size-exclusion HPLC (SE-HPLC) profiles of two recombinant supplements in Figure 7, LEFT (rAlbumin) and Figure 7, RIGHT (rTransferrin) show the purity of those supplements as part of routine product specification testing that makes up the product quality package.

Figure 7: ()
Discussion
Increased cell-derived product requirements historically have been addressed through construction of manufacturing facilities containing large bioreactors dedicated to manufacturing single products. But because of rising operational costs, spiraling cost of goods, excess capacity, and the global financial crisis, such facilities may no longer remain economical. New technologies for large-scale production are being rapidly introduced: e.g., single-use systems and multiproduct facilities. Optimization of output from the upstream cell culture system has also received attention for its potential to increase productivity.
Media development is being investigated in efforts to enhance cellular properties such as growth, viability, and productivity. Regulatory constraints coupled with an increased knowledge of cellular nutrient requirements have encouraged media formulations to evolve from serum-containing to chemically defined, serum-free, and (most recently) protein-free media. Because a protein-free environment is often found to be disadvantageous to cell growth, it requires supplementing with components such as growth factors, amino acids, and albumin.
Our experimental examples show some of the potential benefits that can be gained in designing a media development strategy that involves defined media supplements. Applicable at various stages in biomanufacturing process development, such products can directly replace serum in applications such as cryopreservation, where we found recombinant albumin to provide an animal-free alternative with equivalent or even superior performance.
During production, a chemically defined media environment often requires supplementation to create a robust media platform that can provide unique cell lines with their specific nutrient requirements. Our results indicate that supplementing CD-SFM with a growth factor provided an environment that can sustain higher cell viabilities and reduce apoptotic cell levels, which translated to a significant increase in product titer. Protein-deficient media need help protecting cells in the bioreactor environment, and addition of an animal-free albumin demonstrated potential antioxidant properties.
Mammalian cell-culture is developing as the expression system of choice for other cell-derived products. Our initial studies indicate that a bioactive protein supplement combining growth factors, transferrin, and albumin could provide an animal-free culture environment for sustaining strong growth of commercially relevant vaccine cell lines.
Although product titer is often the primary driver in production of protein therapeutics and emerging cell-based technologies, quality is becoming increasingly important at all stages of manufacturing. QbD concepts are no longer applied purely to manufacturing process parameters for delivering products with consistent quality attributes. Raw material quality is now becoming part of its scope, requiring analytical techniques for testing product consistency and purity. Biomanufacturers should choose raw-material suppliers that can work closely with them during product development, supplying the highest quality materials to suit their needs. That in turn will deliver potential process and performance consistency for patients.
Safe and defined quality media supplements gives process engineers an opportunity to develop chemically defined serum-free media strategies that provide some of the cellular benefits of serum while allowing for robust and reliable media formulations. Use of bioactive proteins is contributing to greater consistency in process performance and providing the scope to elevate process yields.
About the Author
Author Details
Luke Dimasi is a product manager in cell culture for Novozymes Biopharma US Inc., One Broadway, 14th Floor, Cambridge MA 02142, USA; 1-617-401-2500; [email protected]; www.novozymes.com.
REFERENCES
1.) Grillberger, L.. 2009. Emerging Trends in Plasma Free M
anufacturing of Recombinant Protein Therapeutics Expressed in Mammalian Cells. Biotechnol. J. 4:186-201.
2.) van der Valk, J. 2010. Optimization of Chemically Defined Cell Culture Media: Replacing Fetal Bovine Serum in Mammalian In Vitro Methods. Toxicol. in Vitro 24:1053-1063.
3.) Butler, M. 2005. Animal Cell Cultures: Recent Achievements and Perspectives in the Production of Biopharmaceuticals. Appl. Microbiol Biotechnol. 68:283-291.
4.) Jayme, DW, and SR. Smith. 2000. Media Formulation Options and Manufacturing Process Controls to Safeguard Against Introduction of Animal Origin Contaminants in Animal Cell Culture. Cytotechnol. 33:27-36.
5.) Petiot, E. 2010. Rapid Screening of Serum-Free Media for the Growth of Adherent Vero Cells By Using a Small-Scale and Non-Invasive Tool. Appl. Biochem Biotechnol 160:1600-1615.
6.) Butler, M. 2000. Application of a Serum Free Medium for the Growth of Vero Cells and the Production of Reovirus. Biotechnol Prog 16:854-858.
7.) van Wielink, R. 2010. Adaptation of a Madin-Darby Canine Kidney Cell Line to Suspension Growth in Serum-Free Media and Comparison of Its Ability to Produce Avian Influenza Virus to Vero and BHK21 Cell Lines. J. Virol. Meth. 171:53-60.
8.) Buckler, A, and M. Al-Rubeai Iijima, S and K. 2008.Effects of Serum and Growth Factors on HEK 293 Proliferation and Adenovirus ProductivityAnimal Cell Technology: Basic & Applied Aspects, The Netherlands, Springer:19-23.
9.) Horvath, B, M Mun, and MW Laird. 2010. Characterization of a Monoclonal Antibody Cell Culture Production Process Using a Quality By Design Approach. Mol. Biotechnol:1-4.
10.) ICH Q8(R2) Pharmaceutical Development. US Fed. Reg. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2Guideline.pdf 71:53-60.
11.) Grosvenor, S. 2008.The Role of Media Development in Process OptimizationBioPharm Int, An Historical Perspective.
12.) Lanan, M. 2009.QbD for Raw MaterialsQuality by Design for Biopharmaceuticals: Perspectives and Case Studies, Wiley Interscience, Hoboken:193-210.
You May Also Like






