PEGylation of BiologicsPEGylation of Biologics
March 1, 2013
In the 1970s, life-science researchers envisioned protein therapeutics as the ultimate targeted therapy. Companies could use them to address genetic deficiencies and cancer, among other disease classes, as well as to nudge the immune system for treating autoimmune disorders. The first therapeutic proteins were derived from animal or microbial cells, so patients launched immune responses to them that could curtail their activity and produce dangerous side effects.
PEGylation was initially used to prevent immune responses with such drugs. PEG is polyethylene glycol, which typically exists in the form of long polymer chains. Simultaneously hydrophilic and lipophilic, water soluble, and nontoxic, PEG is synthesized from chains of ethylene oxide, which can branch or stretch to any length. Typically, PEG is linked to a protein or peptide through reactive molecular groups on amino acid side chains — most often lysine (Figure 1).
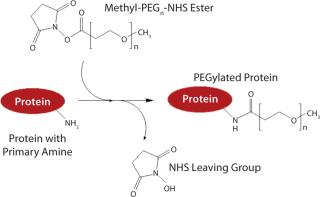
Figure 1: ()
PEG has long been used as an excipient in pharmaceuticals, as well as a base for creams and suppositories. That history of safety made this polymer an attractive choice for countering immune responses with proteins. Its effect on immunogenicity is believed to be tied to protein aggregation, which greatly increases immune response. PEGylation reduces protein aggregation, possibly by physically blocking protein-protein interaction, and thus dampens that response. It also may physically block antibodies and reduce the uptake of antibody-bound molecules by dendritic cells.
As advanced cloning and expression techniques made humanization of proteins possible, immunigenicity was no longer a key issue. But protein therapeutics are susceptible to other biological mechanisms such as proteolytic enzymes and renal clearance. Kidneys filter cells and large proteins from the bloodstream, passing smaller proteins and peptides out in urine (renal clearance). Each ethylene glycol subunit binds two or three water molecules, causing it to swell and giving a PEGylated protein greater size. That ballooning effect allows the protein to pass through the kidney gauntlet, preventing its elimination through urine. The PEGylated structure also helps protect a protein from proteolytic degradation, by which digestive enzymes break down proteins.
PEGylation also has powerful effects on peptides. These short-chained cousins of proteins have a wide range of potential biological activity, making them attractive drug candidates, but they have very short in vivo half-lives for similar reasons as proteins. But their smaller size makes them easier to digest and thus more susceptible to proteolysis, as well as renal clearance. And PEGylation improves their chances of survival, too. Improvements in therapeutic half-life allow lower and less frequent doses, which saves money, improves patient compliance, and reduces the risk of toxicity and allergic reactions.
Proteins that are rapidly cleared must be readministered frequently. But serum levels don’t maintain a steady state. “You get this spiking effect [with periodic high serum protein levels],” says Abraham Abuchowski (chief executive and scientific officer for Prolong Pharmaceuticals), “and that leads to toxicity. But if you can PEGylate it and maintain a low, but steady blood protein level, then you can have very good activity and low toxicity. In pharmaceutical development, you are looking for how much product you can put into [a patient] before toxicity occurs.”
With careful selection, PEGylation may have no effect on the binding or function of a biomolecule, but it often has at least some impact (sometimes a dramatic one). In fact, some conjugates lose activity in vitro because PEGylation can interfere with their binding or enzymatic activity. But a PEGylated protein ultimately may be more therapeutically effective even so because its half-life is dramatically extended. “There are many, many instances in which a protein is PEGylated and has very poor in vitro activity but [has greatly improved] in vivo activity,” says Abuchowski. “Pegasys [Genentech] and Pegintron [Merck] — both of those products have low in vitro activity but superb in vivo activity. That’s a function of the circulating half-life.”
PEGylation is not a simple process (Figure 1). The location and number of PEG conjugations can vary, as can their proximity to binding or active sites on a protein. “You have to adjust the chemistry and/or PEGylation strategy for a particular protein,” Abuchowski points out. “With some proteins — depending on whether they’re interacting with receptors or substrates, and depending on the [tertiary] structure of the protein — you might have to adjust the PEGylation to accommodate that and ensure that the protein maintains [enough of] its in vivo activity.”
“The only technical issue,” he adds, “is how to modify the protein to maintain activity because every single protein is different. You have to know something about the different kinds of PEGs available. And it’s kind of a mix-and-match thing. What’s the appropriate length of the PEG, what’s the appropriate chemistry, where do you want to [place it] on the molecule to make sure you don’t [interfere with receptor binding] or active sites?”
PEGylation first hit the mainstream with the US Food and Drug Administration’s (FDA’s) 1990 approval of Enzon Pharmaceuticals’s Adagen bovine adenosine deaminase, which is used to treat X-linked severe combined immunodeficiency disease (SCID). A full dozen PEGylated products have since been approved, and more are in research and development (R&D) pipelines even now. The worldwide market for PEGylated proteins was about $7 billion in 2012, according to figures from Mountain View Pharmaceuticals. Mark Saifer, the company’s vice president and scientific director, predicts that “sales of PEGylated protein therapeutics will grow faster than the biopharmaceutical market, which itself is growing faster than the pharmaceutical market as a whole. I believe this to be true even though some of the earliest PEGylated protein drugs have attracted the attention of biosimilar developers.”
Gaining Precision
Whether it is applied to proteins, peptides, antibodies, enzymes, or even small molecules, PEGylation involves at least one and sometimes multiple PEG chains grafted to a target. The chain lengths can vary greatly depending on the number of PEG subunits that link up with that molecule. Such variation can cause some product characterization headaches: The resulting product is heterogeneous, and proteins with different extents of PEGylation or different chain lengths are likely to have different physicochemical properties. A number of analytical techniques have been developed specifically to analyze PEGylated proteins, including an enzyme-linked immunosorbent assay (ELISA) kit from Enzo Life Sciences. Thermo Fisher has described a two-dimensional liquid chromatography (LC) system to simultaneously characterize PEGylated proteins and differentiate them from unreacted reagents (1).
A more ambitious approach combines microchip capillary gel electrophoresis (CGE) and a noncommercial high–molecular-weight (HMW) protein a
ssay to create a high-throughput system capable of analyzing the extent of PEGylation in proteins (2). Despite such technical achievements, variability in traditional PEGylation methods can complicate product characterization and manufacturing. Those difficulties have spurred the development of novel chemistries that are intended to achieve PEGylation that exhibits more consistency.
For example, Thermo Fisher and Quanta Biodesign’s dPEG technology uses a discrete PEG with a specific molecular weight. Traditional PEGs have been prepared by a polymerization process that produces a range of polymers of different sizes. The resulting conjugates are similarly diverse, which produces a range of effects on protein/peptide activity and half-life while complicating the manufacturing process. The Quanta Biodesign product comes in linear chains of 4–48 PEG subunits and branched structures of three to nine linear chains.
PolyPEG technology from Polytherics Ltd. uses a poly(methacrylate) backbone to which a series of PEGs are attached, stretching out in parallel like teeth along a comb. Potential benefits include lowered viscosity (e.g., for use with proteins that are administered at high concentration) and the ability to vary both the length of the backbone and PEG “teeth” to fine-tune a resulting conjugate’s properties.
PEG can be single-chained or branched. The latter increases protein solubility, decreases viscosity, and could further increase protein half-life. But it is significantly more expensive, says Ralf Kraehmer (managing director at Celares GmbH, which provides customized PEGylation services). For many proteins, standard, linear PEGylation is sufficient.
Troubles with Lysine: Although PEGs are typically connected to biomolecules using their lysine side chains, in some cases no lysine is structurally accessible. Lysine PEGylation also can be detrimental to some proteins’ efficacy — if that particular amino group is located near a binding or active site region of a molecule. The result of lysine PEGylation could thus be a drastic decrease in a protein’s binding efficiency, according to Bolder Biotechnology, which has actually made some such PEGylated products unsuitable for commercial use.
But many proteins do have several accessible lysines, even at the terminus of their amino acid chains. Some can become multi-PEGylated species and mixed products with different characteristics, which reduces predictability and can lead to manufacturing issues as well as difficulties with product characterization. Such troubles have prompted some companies to move away from lysine as the site of attachment.
Bolder Biotechnology’s strategy is to prevent such randomness through site-specific PEGylation, which introduces PEG to a predetermined location on a protein structure through introduction of a “free” cysteine side chain. Naturally occurring cysteine side chains usually participate in intramolecular disulfide bonds, which prevents them from reacting in PEGylation chemistry. But novel cysteine with no existing disulfide partner would be readily PEGylated. The site of that novel cysteine residue can be carefully selected to prevent interference with biological activity, and the chemistry of PEG itself is altered to be selective for cysteine. Bolder Bio is using this approach with granulocyte-macrophage colony stimulating factor (GM-CSF), which is already approved for melanoma, Crohn’s disease, and myeloid and hematopoietic diseases. The original protein had to be injected daily because of its one-hour half-life, but PEGylation extends that to 22 hours (3).
Ambrx has taken that a step further with its reCODE tehnology, which allows researchers to insert a particular codon (called “Amber”) into any predetermined site in a gene sequence. That provides a means by which the company’s orthogonal tRNA synthetases can then add more than 30 unnatural amino acids that have been selected to be chemically reactive with coupling chemistries used with PEG and other agents (e.g., lipids and other polymers). This technology could be applied to cytokines, peptides, and antibodies for such therapeutic indications as cancer, endocrine disorders, inflammation, and infectious disease.

Table 1: PEGylated products on the US biopharmaceutical market ()
In some cases, random PEGylation is just fine — such as for Enzon’s Oncaspar drug, which is used to treat acute lymphoblatic leukemia. “In the case of enzymes this is the best technical solution,” says Kraehmer, “because you have to cover the whole surface. Otherwise, if you use the single PEGylation or site-specific PEGylation, the protection against the immune system or proteolytic degradation is not sufficient for these huge proteins.”
But single-site PEGylation may be preferred for growth factors and other smaller biomolecules to maintain their biological activity. Analytical characterization is much simpler in this case than with random PEGylation, which produces a mixture of conjugates with a variable number of PEGs attached. As Kraehmer explains, “That means you have to have special steps and some special analytical methods to characterize the PEGylation sites and reproducibility of the PEGylation process.” For example, PEGylation alters the ways in which a protein interacts with separation media. Ion-exchange chromatography, a mainstay of biopharmaceutical separations, becomes more challenging because PEGylated proteins have a much-reduced loading capacity onto ion-exchange resins. According to Kraehmer, that necessitates changes in column size, resin quantity, and volume.
Table 1: PEGylated products on the US biopharmaceutical market

Table 1: PEGylated products on the US biopharmaceutical market ()
Table 2: Selected PEGylated products and technologies in the pipeline
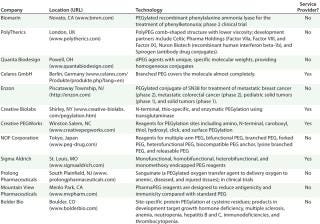
Table 2: Selected PEGylated products and technologies in the pipeline ()
Cost is a key consideration because PEGylation can be an expensive process. Says Abuchowski: “You can get to some very esoteric methodologies, and the question becomes, ‘Can it be scaled up, and can it be done so that the product can be made at a reasonable cost?’”
Not So Nonimmunogenic?
Most companies report that PEGylation is nonimmunogenic, but scientists at Mountain View Pharmaceuticals disagree. “The notion is derived from animal studies that were done with PEG that was not coupled to any therapeutic agent,” counters Mountain View’s Saifer. “So historically, the notion of PEG being nonimmunogenic and nonantigenic became pervasive. That language was used in many patents and publications for many years until it became ingrained in the biopharmaceutical community.” But some early studies indicated that coupling of PEG to a protein could cause it to act like a hapten and draw an immune response in patients (4). “Many people working in the field have basically ignored that and gone ahead,” he says, “continuing in their belief that they were dealing with nonimmunogenic and non-antigenic conjugates.”
One recent clinical example includes the Oncaspar asparaginase drug from Sigma-Tau Pharmaceuticals. A study led by Jonathan Armstrong of the University of Southern California showed that about half the patients treated with PEGylated asparaginase developed antibodies to the conjugate, primarily against the PEG component (5). Further investigation revealed that about half the patients treated showed sufficient antibody titers to accelerate clearance of the conjugate and adversely affect treatment efficacy. “[It] deprives the patient of most of the benefit of the product and in some cases leads to adverse effects that result in the treatment being terminated,” Saifer explains. “So it is clinically important, at least for those multiply PEGylated proteins that are on the market (such as Roche’s Pegasys, Affymax and Takeda’s Omontys, UCB Pharma’s Cimzia, Savient Pharmaceuticals’ Krystexxa, and Oncaspar). How important the antimethoxy PEG antibodies are for monopegylated and dipegylated conjugates is less clear.”
In another example, researchers at Biomarin found that some patients produced anti-PEG antibodies to a PEGylated form of phenylalanine ammonia lyase, which the company is developing for treatment of phenylketonuria cases that do not respond to diet (6). “The extent to which the methoxy group is responsible for that immunization is not completely clear,” Saifer says. “We have heard that there is some role of the methoxy group, but we don’t know the percentage.”
Mountain View researchers have studied the PEG-related immune response in mice and rabbits. “[It varies] from animal to animal,” Saifer reports, “but in general, the methoxy group is a major contributor; perhaps 80% and even up to 95% of the anti-PEG antibodies show selectivity for the methoxy group.”
As an alternative, Mountain View has developed a hydroxy-PEG that appears much less immunogenic, according to Saifer. “It is conceivable that patients who have become immunized to the methoxy PEG will then respond better to and better tolerate hydroxy-PEG conjugates,” Saifer says. “As more PEGylated products enter the market, it becomes more likely that a patient who has received one or another of these drugs would at some other time in life receive a different one. So cross-reaction is a concern. And the evidence is quite clear that when a patient (or an animal) is immunized against methoxy-PEG, those antibodies will react with several other methoxy-PEG conjugates.”
Not everyone is convinced, however. Abuchowski agrees that some patients have an immune response to PEGylation, but “it’s a very small percentage of people. Mountain View claims that its PEG is less immunogenic, and that may be — but I am sure that if it is used as much as the other PEG has been used in patients, then I think you’ll find a subset of patients who make antibodies with that form of PEG as well.”
Applications in Bioprocessing
Experience gained with PEGylation of therapeutics could be applied to other fields, particularly bioprocessing. PEGylation can alter some qualities of proteins or enzymes, making them more soluble in desirable solvents, for example, or granting them greater stability in a variety of conditions. Proteins generally fare poorly in nonaqueous media, but many chemical applications require organic solvents. PEGylation preserved the function of alpha-chymotrypsin, lipase, and catalase in organic solvents (7,8,9). The increased water solubility conferred by PEGylation can also improve its performance in hydrogels (10).
PEGylated proteins have been used in the textile industry, for example limiting the hydrolysis of wool to just its cuticles, giving the resulting fabric shrink resistance and felt-like properties while maintaining its strength and weight (11). In detergents, PEGylation has improved stain-removal properties at low temperatures, which can help preserve fabric color (12). PEGylation also improves thermal stability. Applied to cytochrome C, it raised the maximum peroxidase activity temperature from 80 °C to over °100 C while improving kinetics on a hydrophobic substrate (13). Some have speculated that, due to an ability to improve protein solubility and stability, PEGylation might also find use in bioreactor systems and biosensors.

Protein stability is a key issue for biosensors, because degradation can prevent reproducibility of measurements. PEGylation provides a potential solution. One research group PEGylated cytochrome C in a model biosensor consisting of a self-assembled monolayer of l-cysteine on a polycrystalline, polished gold surface. The biosensor used heme-group electrochemistry and cyclic and square-wave voltammetry. PEGylation of cytochrome C had no effect on its structure or function. The researchers subjected their biosensor to warm storage conditions and found that the PEGylated version had much higher electrochemical response than the unmodified one even after two months of such exposure (14).
In another biosensor application, researchers used PEGylation to modify maltose periplasmic binding protein covalently coupled to NBDamide, which is an environmentally sensitive fluorophore. PEGylation promoted water retention near the protein and reduced potential steric interactions between it and surrounding matrix. The modification led to a significant increase in fluorescence intensity over the unmodified sensor protein (15). Other applications could include microarrays, ELISA tests, and the production of biofoams and biofuels (16).
Future Directions
PEGylation can also bolster existing therapeuti
cs. Biogen-Idec’s Avonex brand is the leading interferon beta 1a product on the market, according to Saifer, and the company has a PEGylated form of it in phase 3 trials. “Making a PEG conjugate could allow patients to take fewer injections and get good efficacy,” he says.
Bayer Healthcare Pharmaceuticals has a PEGylated peptide in its pipeline for treating type 2 diabetes and a PEGylated version of the antibacterial endopeptidase lysostaphin for treating multidrug–resistant strains of Staphylococcus aureus(17, 18). Pro Bono Bio Group has licensed PolyTherics’ TheraPEG platform to develop a TheraPEG–FVIII product. Its formulation is designed for a longer half-life than existing FVIII products have. PBB has already licensed the same technology to develop long-acting forms of Factor IV for treatment of hemophilia B and Factor VIIa for hemophilia A and B as well as trauma applications.
A number of PEGylated therapeutics are coming off patent protection, including Amgen’s Neulasta growth colony-stimulating factor and both Merck’s Pegintron and Genentech’s Pegasys interferon products. Some companies are developing new versions of such therapeutics using newer-generation PEG chemistries that are designed to improve drug characteristics or make them more stable.
“A lot of proteins — recombinant, human and nonhuman — could potentially have therapeutic value or [are already approved and] could be improved by applying the PEGylation strategy,” Abuchowski points out. “And I think that has been where the industry has been going in general when you look at things like Neulasta, PegIntron, and Pegasys.”
PEGylation is usually not required for antibody therapeutics because they are large proteins and therefore cleared slowly by the kidneys. But PEG could improve tumor targeting. And there is a trend towards using antibody fragments and scaffolds because they are cheaper and easier to manufacture. However, they are small enough to have short half-lives, so PEGylation is increasingly being applied to them, according to Kraehmer.
Other potential PEG applications include its use as a linker to increase solubility of hydrophobic cytotoxins in antibody–drug conjugates. It may also be useful in cell therapies. For example, immune responses to therapeutic cells is a key issue in transplantation medicine. PEGylation has been used to help disguise erythrocytes and other cells. It is believed to camouflage antigenic sites and membrane surface charge as well as physically blocking receptor–ligand and cell–cell interactions. PEG also appears to stimulate induction of tolerance due to weak costimulation of alloreactive T cells, which leads them to undergo apoptosis. Studies of PEGylated rat and mouse islet cells suggest that PEG does not interfere with glucose homeostasis signaling processes (19).
Similarly, PEG could help disguise plastic coatings for medical impacts from patients’ immune systems. That comes from its potential to repel cells, which reduces the possibility of infection and undesired tissue growth for implanted devices. Because PEG can be difficult to link to plastic and metal devices alone, some researchers have first linked it to hydrophilic peptides (20).
PEGylation is attractive in part because of its long history of biocompatible applications. Kraehmer says, “PEGylation is one of the most popular formulations because it is already approved by [regulatory] authorities, and you have a lot of examples on the market with low risk. There’s a lot of knowledge compared with some competitive technologies.” Still, PEG is not for everyone. The technology requires additional processing steps, including chemical coupling followed by separation of PEGylated from unreacted proteins and PEG reagents, followed by separating out a subset of PEGylated proteins based on the extent of PEGylation and the position at which it has occurred (if more than one site is possible). “If you can avoid that, of course you will do so,” Kraehmer confirms. “Otherwise PEGylation is the technique of choice.”
Abuchowski agrees: “I haven’t seen any technologies that look like they’re going to displace PEGylation in the near future — or even in the far future. PEGylation works very well. It’s become the gold standard for protein drug delivery. It’s not without its problems, but it’s been very successful. Nine (PEGylated protein) products are approved. Many could not have been approved without it.”
PEGylation has grown to play an integral role in biotechnological research and development, as well as discovery. Abuchoski concludes, “As long as we keep discovering proteins with therapeutic applications, I think there is going to a role for PEGylation.”
About the Author
Author Details
Based in Bellingham, WA, Jim Kling is a freelance science writer with a background in organic chemistry and over a decade of experience covering topics from biotechnology to astrophysics to archeology. His credits include WebMD, The Washington Post, Scientific American, Technology Review, Science magazine, and newsletters of the Harvard Business School. He also occasionally writes science fiction, [email protected], www.nasw.org/users/jkling.
REFERENCES
1.) Crafts, C. Poster: Analytical Methods to Qualify and Quantify PEG and PEGylated Biopharmaceuticals.
2.) Seyfried, BK. 2012. Microchip Capillary Gel Electrophoresis of Multiply PEGylated High–Molecular-Mass Glycoproteins. Biotechnol. J. 7:635-641.
3.) Hu, G. 2010. PEGylating Peptides (and Proteins). BioProcess Int. 8:40-41.
4.) Richter, AW. 1984. Polyethylene Glycol Reactive Antibodies in Man: Titer Distribution in Allergic Patients Treated with Monomethoxy Polyethylene Glycol Modified Allergens or Placebo, and in Healthy Blood Donors. Int. Archs. Allergy Appl. Immun. 74:36-39.
5.) Armstrong, JK. 2007. Antibody Against Poly(Ethylene Glycol) Adversely Affects PEG-Asparaginase Therapy in Acute Lymphoblastic Leukemia Patients. Cancer 110:103-111.
6.). Press release: Highlights from BioMarin’s Research and Development Day.
7.) Castillo, B. 2006. On the Relationship Between the Activity and Structure of PEG-α-chymotrypsin Conjugates in Organic Solvents. Biotechnol. Bioeng. 94:565-574.
8.) Inada, Y. 1986. Application of Polyethylene Glycol-Modified Enzymes in Biotechnology Processes: Organic Solvent-Soluble Enzymes. Trends Biotechnol. 4:190-194.
9.) DeSantis, G, and JB. Jones. 1999. Chemical Modification of Enzymes for Enhanced Functionality. Curr. Opin. Biotechnol. 10:324-330.
10.) Veronese, FM. 2001. Pegylated Enzyme Entrapped in Poly(vinyl alcohol) Hydrogel for Biocatalytic Application. Farmaco 56:541-547.
11.) Schroeder, M. 2004. Chemical Modification of Proteases for Wool Cuticle Scale Removal. Biocatal. Biotransformation. 22:299-305.
12.) Schroeder, M. 2006. Restricting Detergent Protease Action to Surface of Protein Fibres by Chemical Modification. Appl. Microbiol. Biotechnol. 72:738-744.
13.) García-Arellano, H. 2002. High-Temperature Biocatalysis by Chemically Modified Cytochrome C. Bioconjug. Chem. 13:1336-1344.
14.) Santiago-Rodríguez, L.. 2011. Enhanced Stability of a Nanostructured Cytochrome C Biosensor By PEGylation. J. Electroam. Chem. 663:1-7.
15.) Dattelbaum, AM. 2009. PEGylation of a Maltose Biosensor Prom
otes Enhanced Signal Response When Immobilized in a Silica Sol-Gel. Bioconjug. Chem. 20:2381-2384.
16.) González-Valdez, J. 2012. Advances and Trends in the Design, Analysis, and Characterization of Polymer–Protein Conjugates for “PEGylaided” Bioprocesses. Anal. Bioanal. Chem. 403:2225-2235.
17.) Tom, I. 2009. Reproducible Production of a PEGylated Dual-Acting Peptide for Diabetes. AAPS J. 9:E227-E234.
18.) Walsh, S. 2003. Improved Pharmacokinetics and Reduced Antibody Reactivity of Lysostaphin Conjugated to Polyethylene Glycol. Antimicrob. Agents Chemother. 47:554-558.
19.) Scott, MD. 2004. Beyond the Red Cell: Pegylation of Other Blood Cells and Tissues. Transfus. Clin. Biol. 11:40-46.
20.) Kenan, DJ. 2006. Peptide–PEG Amphiphiles As Cytophobic Coatings for Mammalian and Bacterial Cells. Chem. Biol. 13:695-700.
You May Also Like






