Lessons in Bioreactor Scale-Up, Part 4: Physiochemical Factors Affecting Oxygen Transfer and the Volumetric Mass-Transfer Coefficient in Stirred TanksLessons in Bioreactor Scale-Up, Part 4: Physiochemical Factors Affecting Oxygen Transfer and the Volumetric Mass-Transfer Coefficient in Stirred Tanks
October 15, 2024
Previous installments of this series throughout 2024 have discussed the basics of bioreactor scaling, mixing, and rates of oxygen uptake and mass transfer, along with the most commonly applied dynamic method for mass-transfer measurement. This month, part 4 continues the discussion of the volumetric mass-transfer coefficient (kLa) by showing how different culture and bioreactor operating parameters can affect its value. The next installment of this series will begin 2025 by presenting some theoretical correlations described in published literature that are used to determine kLa and often used in bioreactor scale-up exercises.
(see The Series So Far box).
Gas Sparging and Oxygen Supply
Bioreactors are used for suspension-culture–based manufacture of most modern biopharmaceuticals. In such processes, oxygen is a key nutrient for cell growth, culture maintenance, and protein production. The typical oxygen uptake rate value for mammalian cell cultures at a concentration of ≈106 cells/mL is 0.05–1.5 mmol O2 per liter of culture per hour. The kLa value is a key performance parameter, representing a given bioreactor’s capacity for oxygen supply and efficiency of transfer from introduced gases into liquid solution. Such measurement is important because living cells can metabolize oxygen only when it is dissolved in solution. To enable proper cell metabolism, oxygen must be supplied continuously to make sufficient dissolved oxygen (DO) available to every point in the vessel.
Gaseous oxygen in suspended air bubbles takes up to eight steps to transit through the gas, reach liquid medium, and become available intracellularly for energetic transformations (Figure 1). Resistance to mass transfer can be encountered at different points throughout this process:
• transfer from the bulk gas phase (gas-bubble interiors) to the gas–liquid interface
• transfer across the gas–liquid interface
•diffusion through relatively stagnant liquid films
• transport through bulk liquid
• diffusion through relatively stagnant liquid films around cells
• movement across the liquid–cell-surface interface
• for clumps (flocs) of cells or those attached to microcarriers, diffusion across the solid to an individual cell
• transport through the cytoplasm to the consumption site (mitochondria).
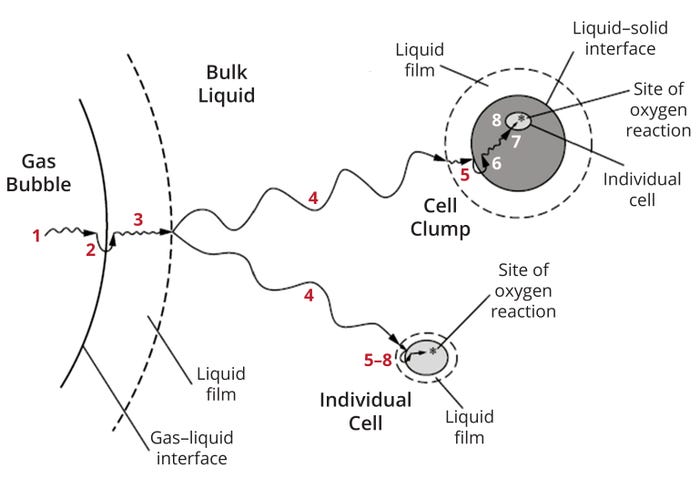
Figure 1: Mass-transfer steps (and resistances) encountered during oxygen transfer from a gas phase to an individual cell or a cellular floc (1).
Not all transport resistances encountered during those steps are equal in magnitude, and some can be neglected depending on conditions prevailing in a given culture. Because oxygen solubility in an aqueous solution is low, liquid films offer the most resistance to mass transfer and thus are key determinants of the value of kLa.
The most practical means of supplying oxygen to a suspension cell culture is by blowing air into the bioreactor through a sparger. The sparger generally is placed under the mixing impeller so that the blade’s rotation will break up bubbles and entrench them into the liquid medium. Those bubbles then take a tortuous path to move through the liquid layers and eventually burst after reaching the surface of the culture. The liquid height in a small-scale bioreactor with a height/diameter (H/D) ratio close to 1:1 generally will be small, so air bubbles reach the top and emerge rather quickly, making the efficiency of oxygen transfer for a given air-flow rate relatively low. However, as bioreactor scale increases, so do liquid height (H/D > 1) and the holding time of air in the culture. That in turn increases the efficiency of oxygen transfer.
Factors Affecting Oxygen Transfer Rate and kLa
In stirred-tank reactors, air supply is optimized to provide maximum gas transfer through the use of shear, power, and turbulence to maximize the ratio of gaseous surface area to liquid volume and gas velocity. The oxygen transfer rate (OTR) of a bioreactor is strongly influenced by the hydrodynamic conditions prevailing in the vessel. Those conditions are a function of turbulent kinetic energy dissipation. Thus, the kLa value is influenced by a number of factors: process parameters (agitator speed, temperature, pH, and gassing rate), physiochemical characteristics of the culture medium (viscosity, density, salt content, surface tension, and coalescence behavior), biochemical parameters (cell concentration and OUR), and equipment geometries (vessel, agitator, and sparger). Figure 2 illustrates the interrelationship of such factors. Their influence can change the value of either the liquid mass-transfer coefficient (kL), the interfacial area (a), and/or the driving force for mass transfer (C* – C, where C represents oxygen concentration and C* is the oxygen-saturation concentration).
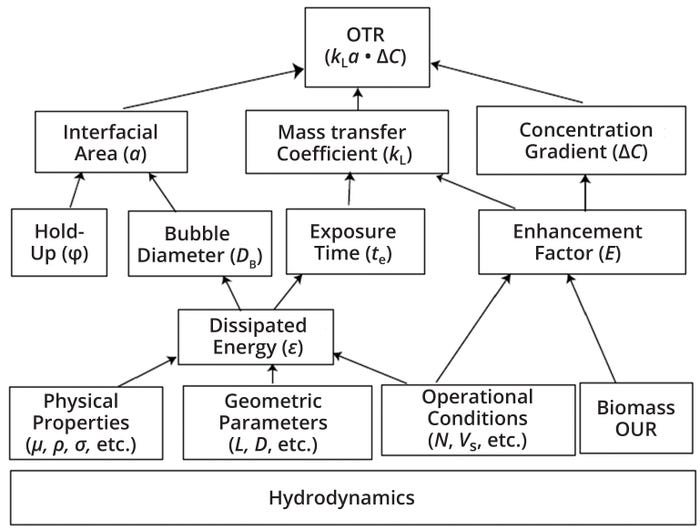
Figure 2: Interrelationship of bioprocess variables with oxygen transfer rate (OTR) and kLa (11); OUR = oxygen uptake rate, μ = coefficient of friction, ρ = fluid density, σ = surface tension, L = length, D = diameter, N = impeller rotational speed, Vs = gas superficial velocity.
Gas Bubbles: The behavior of the gas bubbles inside a bioreactor exerts a strong influence on the overall value of kLa. Some properties of gas bubbles affect the magnitude of kL; others change a.
The most important property of the gas bubbles is their size. For a given volume of gas, more interfacial area will be created if it is dispersed into many small bubbles rather than a few large bubbles (1). Small bubbles have slow bubble-rise velocities and hence spend more time in the liquid, allowing more time for oxygen to dissolve (and CO2 to be adsorbed from the liquid). Therefore, small bubbles lead to higher gas hold-up (the fraction of the bioreactor working volume that is occupied by the gas).
During gas sparging in a bioreactor, bubbles either break up into smaller bubbles or merge together with others to form larger bubbles. Both processes — bubble break-up and coalescence — greatly influence the gas-bubble size distribution and hence the mass-transfer area. Some practical considerations, however, constrain the size of gas bubbles in a bioreactor. Those much smaller than 1 mm in diameter often cause foam formation on the liquid surface, which is undesirable for many reasons. In addition, the rising velocity of those very small bubbles is slow enough that oxygen (and CO2) concentration in them can equilibrate quickly with that of the liquid medium, eliminating their function for transferring gas into and out of the liquid.
Small bubbles generally are beneficial, however, providing higher gas hold-up and interfacial area than larger bubbles. The optimal bubble diameter for mass transfer in a bioreactor is 2–3 mm. Beyond 3-mm size, any further increase in kLa will be minimal.
Bubble Break-Up: With bioreactors in which turbulent flow generally prevails, four mechanisms have been identified to cause bubble break-up (2): turbulent fluctuations and collisions, viscous shear stress, shearing-off processes, and interfacial instability. Mathematical modeling of bubble break-up typically includes two submodels: one for the frequency of break-ups and the other for the resulting “daughter”-bubble size distribution. Most models assume a simple and equilateral binary break-up (3, 4).
Bubble Coalescence: Coalescence of small bubbles into larger ones is generally undesirable because it reduces the total interfacial area and gas hold-up, thus limiting oxygen mass transfer. The phenomenon of bubble coalescence is more complicated than that of bubble break-up, with several published theories and mathematical models describing the process (5–9). Pure water is a coalescing liquid, but the presence of solutes (such as salt or ions) in it renders it noncoalescing.
Because cell-culture media contain salts and other organic compounds, they behave to a certain extent as noncoalescing liquids, which is advantageous for oxygen transfer. The addition of ions to water in sparged vessels markedly reduces the average bubble size overall and increases the gas hold-up, leading to as much as 10× greater interfacial area than that obtained without salts (10). Because the composition — and therefore the coalescence properties — of a highly dense fed-batch cell-culture broth vary over time, kLa also can be expected to vary accordingly.
Sparger Design: The biopharmaceutical industry uses several different types of spargers in bioreactors for cell culture, from sintered spargers that generate μm-sized bubbles to drilled-hole or open-pipe (perforated) spargers that have defined numbers and sizes of holes to generate precisely sized bubbles. In a stirred-tank bioreactor, the bubbles leaving the sparger usually fall within a narrow size range. However, impeller rotation and the resulting turbulence cause dispersion, with continual break-up and coalescence of bubbles taking place. Hence, the actual bubble size in such a bioreactor is quite different from what the sparger emits.
Gas Dispersion: Two-phase gas–liquid flow in stirred tanks has been studied extensively and is well documented (11). Gas dispersion and bubble size — and the residence time of bubbles in a bioreactor — both depend on impeller type and location as well as stirring speed. The kLa values generally will increase along with impeller tip speed. Balancing impeller rotation and gas-flow rate determines the effectiveness of bubble break-up and dispersion. At sufficiently high stirrer speeds, gas will disperse and be recirculated around the tank and thus contribute to mass transfer. Similarly, impeller design has a strong influence on oxygen mass transfer, with Rushton turbines proven to be more effective in generating higher gas dispersion and bubble break-up than pitched-blade impellers (1, 11).
Physiochemical Properties of the Liquid: The physiochemical properties of a fluid affect oxygen transfer through it due to their significant effects on bubble size, gas hold-up, and hence kLa. Equation 1 below correlates kLa with fluid density (ρ) and viscosity (μ) (12):
Equation 1
kLa = 37.5 (ρ/μ)0.667
The inverse correlation of kLa with viscosity in Equation 1 is due to the fact that with increasing viscosity, the thickness of liquid film surrounding each gas bubble increases. High viscosity also dampens turbulence and reduces the effectiveness of gas dispersion. An increase in culture-broth density, on the other hand, positively influences kLa. Figure 3 clearly shows the effects of the coalescing behavior of liquids and of viscosity on kLa. A 5% Na2SO4 solution is noncoalescing, so it yields higher kLa values with the same power input than water (a coalescing liquid), even though both solutions have similar viscosity. Carboxymethyl cellulose is highly viscous, thus yielding very low kLa values even with very high stirrer power input.
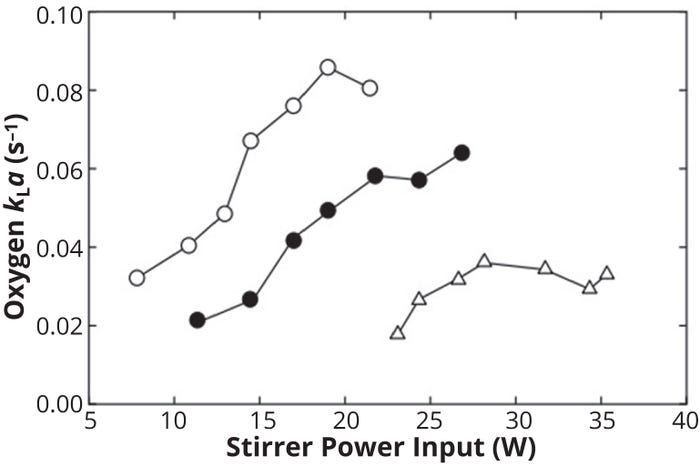
Figure 3: Effect of solution behavior and viscosity on kLa in a stirred-tank reactor; ○ = 5% sodium sulfate in water (low viscosity, noncoalescing), ● = water (low viscosity, coalescing), = 0.7% carboxymethyl cellulose in water (viscous) (11).
Air-Flow Rate and Stirrer Speed: Increasing the air-flow rate also improves mass transfer and increases kLa values, but only to a certain extent (Figure 4, top panel). In most cases, the increase of kLa with higher gas-flow rates is limited as those flow rates overwhelm the impeller — a condition known as flooding — making it unable to distribute gas in the tank. In addition, very high gas-flow rates can blow liquid contents out of a bioreactor tank.
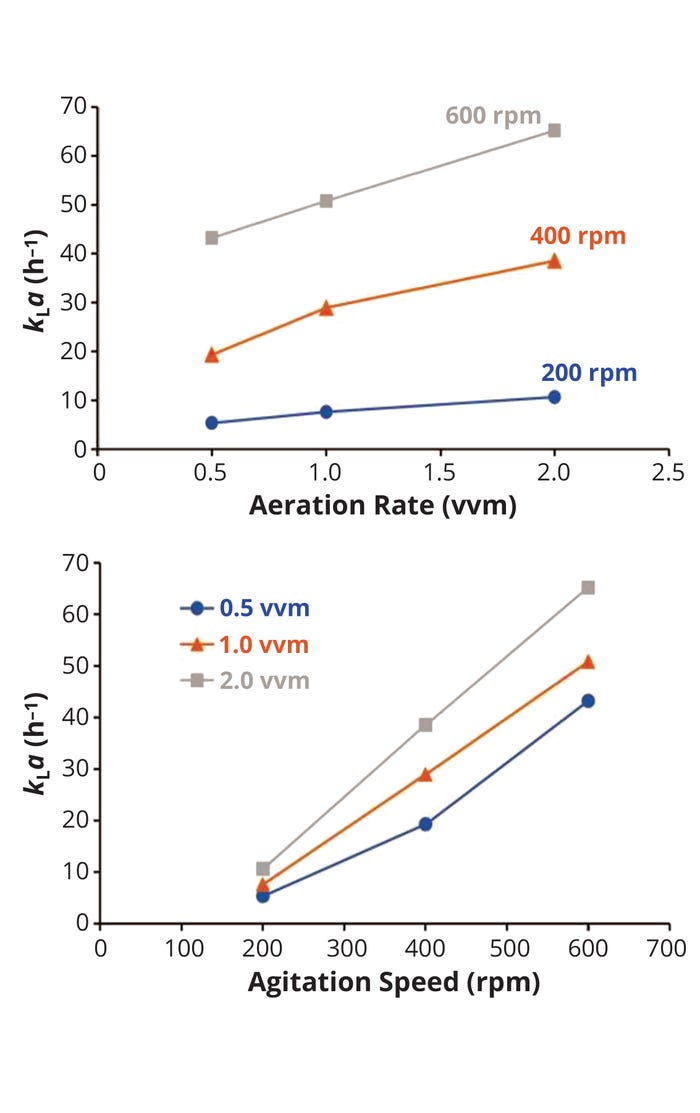
Figure 4: Effect of aeration rate (top) on the volumetric mass transfer coefficient (kLa) at different agitation speeds and of agitation speed (bottom) on kLa at different aeration rates
Source: Abdella A, Segato F, Wilkins MR. Optimization of Process Parameters and Fermentation Strategy for Xylanase Production in a Stirred Tank Reactor Using a Mutant Aspergillus nidulans Strain. Biotechnol. Rep. 26, 2020: e00457; https://doi.
org/10.1016/j.btre.2020.e00457.
Alternatively, increasing the impeller stirring speed (power input) generally yields higher kLa values (Figure 4, bottom panel). Higher power input increases turbulence intensity, which in turn produces smaller bubbles, increases gas hold-up, and improves overall gas distribution. Those effects generally provide significant improvements in oxygen mass transfer and kLa, as discussed above.
Temperature and Pressure: Liquid temperatures in cell-culture bioreactors and aerobic fermentations have opposing effects on oxygen-saturation concentration (C*) and mass-transfer coefficient (kL) (1). Increasing temperatures lower oxygen solubility in water, thus reducing the driving force (C* – C). At the same time, oxygen becomes more diffusive in water, which increases kL. The net effect depends on the range of temperatures considered. Between 10 and 40 °C, an increase is likely to improve the rate of oxygen transfer; beyond 40 °C, the solubility of oxygen drops significantly, causing adverse effects on both driving force and mass transfer.
The total pressure at which a bioreactor (or fermentor) is operated also affects the solubility of gases in liquid culture broth. The partial pressure of a gas is related to its saturation concentration in liquid by means of Henry’s law, which states that the partial pressure of a gas is equal to the total pressure times the mole fraction of gas (Henry’s constant × saturation concentration) in liquid, or mathematically
Equation 2
pO2 = PT × yO2 = H C*O2
where pO2 is the partial pressure of oxygen, PT is total pressure, yO2 is the mole fraction of oxygen, and C*O2 is saturated oxygen concentration in liquid.
Depending on the oxygen demand of cells in bioreactor cultures, it may be necessary to provide them with oxygen-enriched air or pure oxygen rather than air. Equation 3 below expresses how using pure oxygen affects the concentration driving force for mass transfer. Regular air is 21% oxygen, so its mole fraction of oxygen is 0.21; if pure oxygen is used, then the mole fraction is 1.
Equation 3

Equation 3 above compares the concentration driving force (C*) for two sparged gases, one regular air and the other pure oxygen. Hence, sparging pure oxygen at the same temperature and pressure results in nearly fivefold higher oxygen concentration in liquid than regular air can provide. Similarly, oxygen solubility can be increased by using compressed air at higher total pressure (PT). However, using either pure oxygen or compressed air substantially increases the operating cost of a bioreactor.
Shear Protection and Antifoam Agents: Media additives for shear protection (e.g., Pluronic F-68 detergent, PF68, also known as poloxamer 168) and antifoam agents can have a profound effect on oxygen mass transfer and fluid dynamics in a bioreactor. The efficiency and effectiveness of PF68 as a shear-protective agent is well documented (13–17). Reports on its influence on mass-transfer characteristics in bioreactors have been mixed, with some studies showing a reduction (17, 18) and others reporting a significant increase (19, 20) in mass transfer. Media components and additives that can influence culture viscosity and surface-tension properties often are present as well, potentially masking the effects of PF68 in some studies.
Figure 5 illustrates results from a comprehensive study of how different concentrations of PF68 influence kLa in a 400-L stirred tank using different sparger systems (13). The experiments were conducted with a 60-W/m3 power input and an aeration rate of 20 L/min. The results clearly indicate that PF68 affects mass transfer significantly. At very low PF68 concentrations (0.02 g/L), kLa values immediately declined at least 50% compared with a reference (without PF68) with all tested sparger systems. That PF68 concentration is far below the accepted critical micelle concentration (CMC) of 0.33 g/L. Note that further increases in PF68 concentration significantly increased kLa with sintered spargers: Even at 1 g/L PF68, the kLa value for the sparger with the smallest pores (20–47 μm) was similar to that of the reference. No such initial decline and later recovery of kLa as a function of PF68 concentration was observed with either a ring sparger or an open tube.
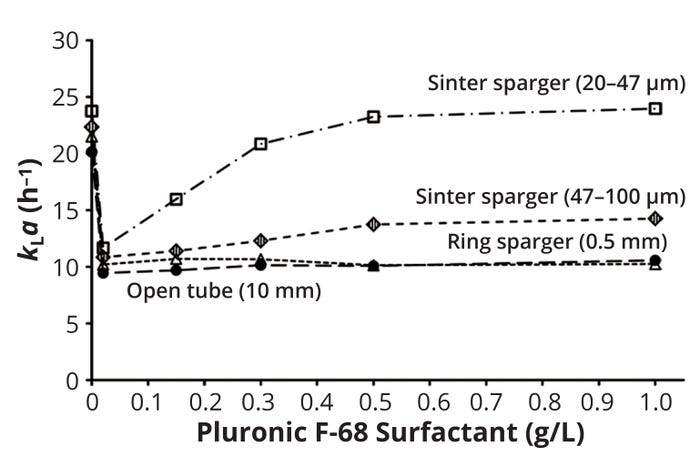
Figure 5: kLa as a function of the Pluronic F-68 detergent concentrations in a 400-L stirred tank with different sparger systems (13).
Foam forms in bioprocesses through the introduction of gases into culture broth and is further stabilized by proteins from the cells in culture. Adding antifoam agents to culture media is the most common method of preventing foam build-up. Both positive and negative effects of antifoams on oxygen transfer have been observed in fermentation-based bioprocesses. In one study, a silicone-based antifoam negatively affected the mass transfer coefficient, gas hold-up, and gas velocity within media (21). Another report, however, showed that antifoams without silicone oil had no great effect on the rate of oxygen transfer, whereas those containing silicone oil did affect it significantly at the beginning of a process, an effect that decreased over the duration (22). Few studies have been published on the effects of antifoam addition on oxygen transfer or kLa of cell-culture systems. In one basic fed-batch study using Chinese hamster ovary (CHO) cells, researchers used bioreactor off-gas analysis by mass spectrometry to determine OUR and kLa values (23). They found that antifoam additions caused an increase in the former and a corresponding decrease in the latter.
Biomass and Macromolecules: Oxygen transfer to a culture broth also is influenced by the presence of cells themselves. Studies of microbial fermentations have shown the presence of microorganisms to enhance the OTRs in the cultures (2–5). Their consumption of oxygen increases the driving force by maintaining a low oxygen concentration in the medium. Microbe-derived variation in the OTR for a given driving force and interfacial area generally is characterized by means of an enhancement factor, E. It is defined as the ratio of mass-transfer flux of oxygen in the presence of microorganisms to the oxygen flux in their absence (11).
Summary: Stirred-tank bioreactors are used widely in bioprocessing (e.g., aerobic fermentation and cell-culture–based production of therapeutic proteins, among others). Such bioreactors provide high rates of mass and heat transfer with excellent mixing. Many variables affect mass transfer and mixing in stirred-tank systems, but the most important among them are stirrer speed, type, and number as well as gas-flow rate. Hence, possible ways to optimize (increase) kLa and OTR include
• increasing power input and/or gassing rate
• optimizing bioreactor design and agitator geometry
• changing the composition of culture media.
Scale-Up Based on Equal kLa
Keeping kLa constant across scales has been used as a criterion for successful scale-up of many microbial fermentation bioprocesses. Their oxygen demands generally are high, and it is well known that oxygen gas–liquid mass transfer is the most significant factor for scale-up of aerobic fermentations. In fact, nearly a third (30%) of microbial fermentation scale-up operations are based on keeping kLa constant (24). The approach has been used in production of antibiotics (24), exopolysaccharides (26), and secondary metabolites (24, 27), as well as Escherichia coli–based production of recombinant proteins (28).
In one study, for example, a secondary-metabolite production process based on aerobic fermentation was scaled up from laboratory (5 L) to pilot scale (50 L) by keeping kLa constant (24). The goal was to provide nearly equivalent kLa values to each fermentor by manipulating agitation and aeration rates, thereby ensuring similar oxygen transfer at both scales. Figure 6 compares the kLa values obtained for each system. In the 5-L fermentor, the highest productivity was obtained with 200-rpm agitation and 1.5-vvm aeration, resulting in a kLa value of 0.02 s–1. To maintain a constant kLa, the authors chose a combination of 180 rpm and 0.5 vvm for the 50-L fermenter (indicated by arrows in Figure 6). Using those values in the 50-L fermentor provided very similar productivities and profiles of cell growth, substrate consumption, DO, and pH — thereby indicating a successful scale-up.
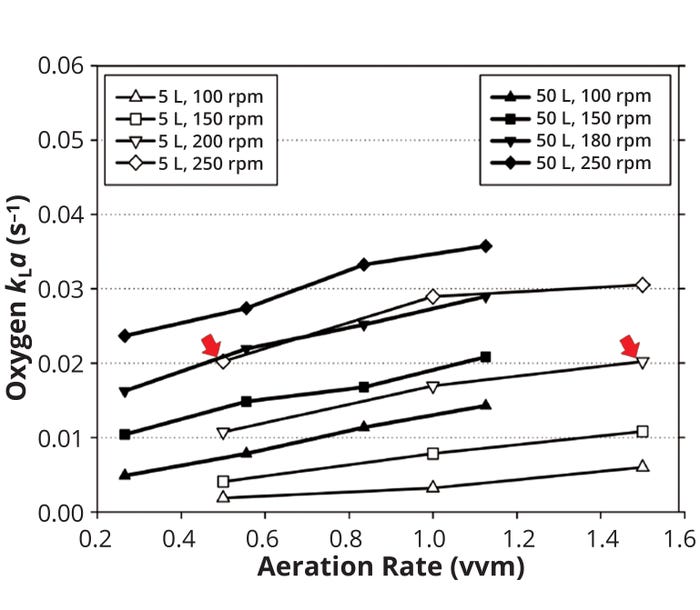
Figure 6: Comparing kLa at 5-L laboratory scale and 50-L pilot scale in stirred-tank bioreactor systems (24).
The Xcellerex (XDR) single-use bioreactor system for cell-culture–based bioprocessing is available from Cytiva in a range of sizes from 10-L to 2000-L volumes. That makes it possible to develop a bioprocess at laboratory scale (10 L) and scale it up to pilot (500 L) then clinical and commercial manufacturing (1000–2000 L) scales. The supplier has published engineering characterization documents (with mixing times, power inputs, and oxygen-transfer capabilities, including achievable kLa values) for each XDR bioreactor model (29–31). Figure 7 compares kLa values achievable using a microsparger (2-μm pore size) in XDR-10, XDR-500, and XDR-1000 systems. Those bioreactors respectively represent laboratory, pilot, and clinical/commercial manufacturing scales for cell-culture processes.
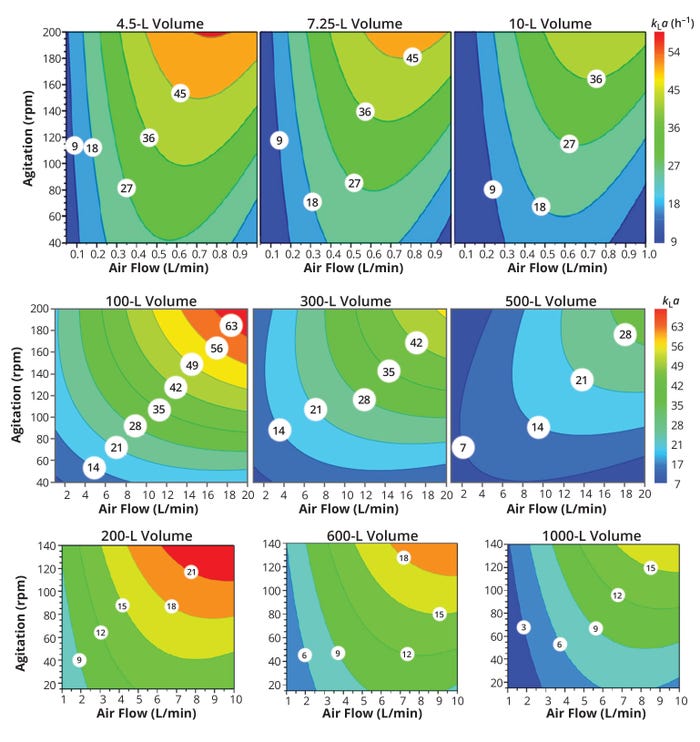
Figure 7: Four-dimensional (4D) response contour plots of kLa representing different air-flow rates, working volumes, and agitation speeds for Cytiva Xcellerex XDR-10 (top row), XRD-500 (middle row), and XDR-1000 (bottom row) bioreactor systems from Cytiva with 2-μm microsparger pore size (29–31).
It is evident that the value of kLa rises with increasing agitation and air-flow rates. However, the XDR bioreactor working volume affects the achievable kLa value negatively, with the achievable value decreasing substantially as working volume increases with a 2-μm pore-size microsparger installed. With a bottom mounted single impeller, however, increasing the working volume with a given agitation rate would lower the volumetric power input to the liquid, thus decreasing turbulence intensity and potentially increasing the frequency of bubble coalescence. All those factors combine to lower both kL and a and thus kLa . In a XDR-10 bioreactor, for example, a kLa value of 27 h–1 is achieved at a much lower combination of agitation and air-flow rate with a working volume of 4.5 L than would be required to achieve the same value at the full working volume of 10 L.
Process engineers applying equal kLa as a scale-up criterion across the XDR platform using the contour plots in Figure 7 from XDR-10 to XDR-1000 systems with 2-μm pore-size microspargers would need to begin by choosing a working volume. In the figure, note that the kLa value that aligns with your needed agitation and aeration-flow rates. Next, choose a working volume for the large-scale bioreactor (XDR-1000), and from the contour plot that also contains the value of required kLa (from the XDR-10 scale), you can determine the necessary agitation and aeration flow rates for the XDR-1000 culture. Then you can operate the large-scale bioreactor at your chosen agitation and aeration flow rates to check the reproducibility of cell-culture performance and product-quality attributes across scales.
Because working volume can have such a profoundly negative influence on the values of kLa in XDR bioreactors, it is possible that some values achievable at small scale might not work at large scale. For example, the kLa value of 18 h–1 achievable in a Cytiva Xcellerex XDR-10 bioreactor operating at its full working volume of 10 L cannot be attained in XDR-1000 system at its full working volume with any combination of agitation and aeration rates.

THE SERIES SO FAR
Part 1 — Exploring Introductory Principles. BioProcess Int. 22(1–2) 2024: 22–27; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scale-up-part-1-mdash-exploring-introductory-principles.
Part 2: A Refresher on Fluid Flow and Mixing. BioProcess Int. 22(6) 2024: 32–40; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scale-uppart-2-a-refresher-on-fluid-flow-and-mixing.
Part 3: Experimental Determination and Application of Oxygen Mass-Transfer Rate, Mass-Transfer Coefficient, and Oxygen- Uptake Rate. BioProcess Int. 22(9) 2024: 26–33; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scaleup-part-3-experimental-determinationand-application-of-oxygen-mass-transferrate-mass-transfer-coefficient-andoxygen-uptake-rate.

References
1 Doran, PM. Chapter 10: Mass Transfer. Bioprocess Engineering Principles, Second Edition. Elsevier: Oxford, UK, 2013.
2 Seidel S, et al. Oxygen Mass Transfer in Biopharmaceutical Processes: Numerical and Experimental Approaches. Chemie Ingenieur. Technik. 93(1–2) 2021: 42–61; https://doi.org/10.1002/cite.202000179.
3 Chu P, et al. A Review of Bubble Break-Up. Adv. Colloid Interface Sci. 270, 2019: 108–122; https://doi.org/10.1002/cite.202000179https://doi.org/10.1002/cite.202000179.
4 Liao Y, Lucas D. A Literature Review of Theoretical Models for Drop and Bubble Break-up in Turbulent Dispersions. Chem. Eng. Sci. 64(15) 2009: 3389–3406; https://doi.org/10.1002/cite.202000179.
5 Chesters AK. The Modelling of Coalescence Processes in Fluid–Liquid Dispersions: A Review of Current Understanding. Chem. Eng. Res. Des. 69(A4) 1991: 259–270.
6 Shinnar R, Church JM. Statistical Theories of Turbulence in Predicting Particle Size in Agitated Dispersions. Indust. Eng. Chem. 52(3) 1960: 253–256; https://doi.org/10.1021/ie50603a036.
7 Howarth WJ. Coalescence of Drops in a Turbulent Flow Field. Chem. Eng. Sci. 19(1) 1964: 33–38; https://doi.org/10.1016/0009-2509(64)85003-X.
8 Lehr F, Millies M, Mewes D. Bubble-Size Distributions and Flow Fields in Bubble Columns. AIChE J. 48(11) 2002: 2426–2443; https://doi.org/10.1002/aic.690481103.
9 Liao Y, Lucas D. A Literature Review on Mechanisms and Models for the Coalescence Process of Fluid Particles. Chem. Eng. Sci. 65(10) 2010: 2851–2864; https://doi.org/10.1016/j.ces.2010.02.020
10 van’t Riet K. Review of Measuring Methods and Results in Non-Viscous Gas–Liquid Mass Transfer in Stirred Vessels. Ind. Eng. Chem. Process Des. Dev. 18(3) 1979: 357–364; https://doi.org/10.1021/i260071a001.
11 Garcia-Ochoa F, Gomez E. Bioreactor Scale-Up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 27(2) 2009: 153–176; https://doi.org/10.1021/i260071a001.
12 Lee J. Development of a Model To Determine Mass Transfer Coefficient and Oxygen Solubility in Bioreactors. Heliyon. 3(2) 2017: e00248; https://doi.org/10.1016/j.heliyon.2017.e00248.
13 Sieblist C, Jenzsch M, Pohlscheidt M. Influence of Pluronic F68 on Oxygen Mass Transfer. Biotechnol. Prog. 29(5) 2013: 1278–1288; https://doi.org/10.1002/btpr.1770.
14 Murhammer DW, Goochee CF. Sparged Animal Cell Bioreactors: Mechanism of Cell Damage and Pluronic F-68 Protection. Biotechnol. Prog. 6(5) 1990: 391–397; https://doi.org/10.1021/bp00005a012.
15 Papoutsakis ET. Media Additives for Protecting Freely Suspended Animal Cells Against Agitation and Aeration Damage. Trends Biotechnol. 9(1) 1991: 316–324; https://doi.org/10.1016/0167-7799(91)90102-n.
16 Palomares LA, González M, Ramírez OT. Evidence of Pluronic F-68 Direct Interaction with Insect Cells: Impact on Shear Protection, Recombinant Protein, and Baculovirus Production. Enz. Microb. Technol. 26(5–6) 2000: 324–331; https://doi.org/10.1016/s0141-0229(99)00176-3.
17 Murhammer DW, Pfalzgraf EC. Effects of Pluronic F-68 on Oxygen Transport in an Agitated, Sparged Bioreactor. Biotechnol. Techn. 6(3) 1992: 199–202.
18 Lavery M, Nienow AW. Oxygen Transfer in Animal Cell Culture Medium. Biotechnol. Bioeng. 30(3) 1987: 368–373; https://doi.org/10.1002/bit.260300307.
19 Lee S-Y, Kim D-I. Effects of Pluronic F-68 on Cell Growth of Digitalis lanata in Aqueous Two-Phase Systems. J. Microbiol. Biotechnol. 14(6) 2004: 1129–1133; https://www.jmb.or.kr/journal/view.html?volume=14&number=6&spage=1129.
20 Toye D, et al. Influence of Medium Composition on Oxygen Transfer Rate in Animal Cell Culture. Canadian J. Chem. Eng. 88(4) 2010: 671–676; https://doi.org/10.1002/cjce.20302.
21 Al-Masry WA. Effects of Antifoam and Scale-Up on Operation of Bioreactors. Chem. Eng. Processing 38(3) 1999: 197–201; https://doi.org/10.1016/S0255-2701(99)00014-8.
22 Koch V, et al. Effect of Antifoam Agents on the Medium and Microbial Cell Properties and Process Performance in Small and Large Reactors. Proc. Biochem. 30(5) 1995: 435–446; https://doi.org/10.1016/0032-9592(94)00029-8.
23 Goh H-Y, et al. Applications of Off-Gas Mass Spectrometry in Fed-Batch Mammalian Cell Culture. Bioprocess Biosyst. Eng. 43(3) 2020: 483–493; https://doi.org/10.1007/s00449-019-02242-2.
24 Shin W-S, et al. Application of Scale-Up Criterion of Constant Oxygen Mass Transfer Coefficient (kLa) for Production of Itaconic acid in a 50-L Pilot-Scale Fermentor by Fungal Cells of Aspergillus terreus. J. Microbiol. Biotechnol. 23(10) 2013: 1445–1453; https://doi.org/10.4014/jmb.1307.07084.
25 Wang Y-H, Zhang X. Influence of Agitation and Aeration on Growth and Antibiotic Production By Xenorhabdus nematophila. World J. Microbiol. Biotechnol. 23(2) 2007: 221–227; https://doi.org/10.1007/s11274-006-9217-2.
26 Bandaiphet C, Prasertsan P. Effect of Aeration and Agitation Rates and Scale-Up on Oxygen Transfer Coefficient, kLa, in Exopolysaccharide Production from Enterobacter cloacae WD7. Carbohydrate Polym. 66(2) 2006: 216–228; https://doi.org/10.1016/j.carbpol.2006.03.004.
27 Herbst H, Schumpe A, Deckwer W-D. Xanthan Production in Stirred Tank Fermenters: Oxygen Transfer and Scale-Up. Chem. Eng. Technol. 15(6) 1992: 425–434; https://doi.org/10.1002/ceat.270150610.
28 Islam RS, et al. Scale-Up of Escherichia coli Growth and Recombinant Protein Expression Conditions from Microwell to Laboratory to Pilot Scale Based on Matched kLa. Biotechnol. Bioeng. 99(5) 2008: 1128–1139; https://doi.org/10.1002/bit.21697.
29 Application Note: 29242383 AA. Engineering Characterization of the Single-Use Xcellerex XDR-10 Stirred-Tank Bioreactor System. Cytiva Inc.: Marlborough, MA, 2020.
30 Application Note: KA4969250918AN. Engineering Characterization of the Single-Use Xcellerex XDR-500 Stirred-Tank Bioreactor System. Cytiva Inc.: Marlborough, MA, 2020; https://cdn.cytivalifesciences.com/api/public/content/digi-18411-pdf.
31 Application Note: 29242384 AA. Engineering Characterization of the Single-Use Xcellerex XDR-1000 Stirred-Tank Bioreactor System. Cytiva Inc.: Marlborough, MA, 2020; https://cdn.cytivalifesciences.com/api/public/content/digi-18517-pdf.
A seasoned industry professional with a PhD in chemical and biological engineering from the University of British Columbia (Vancouver) in Canada and expertise in embryonic stem cell bioengineering, upstream biological process development, and fed-batch bioreactors, Muhammad Arshad Chaudhry is associate director of process and formulation development at Mural Oncology, 852 Winter Street, Waltham, MA 02451; [email protected].
You May Also Like






