ActiCHO Media SystemActiCHO Media System

We have previously reported rapid, consistent, and highly productive fed-batch processes from shake flasks to 5-L glass and 200-L single-use bioreactors using the ActiCHO Media System (1). This system has demonstrated to be highly reliable in many processes using different Chinese hamster ovary (CHO) cell clones. PAA Laboratories/GE Healthcare manufactures this medium in both liquid and powder formats and supplies it worldwide for large-scale applications. Here we present some data on industrial scale-up studies going directly from small shake flasks to 1,000-L bioreactors.
Features of ActiCHO Media System
ActiCHO Media System is chemically defined and therefore entirely free of animal-component ingredients. The system does not contain growth factors, proteins, peptides, or hydrolysates. Most important, it was designed to be a ready-for-use platform and to require no optimization with CHO DG44 cells expressing heterologous genes. This was achieved by formulating a highly balanced set of ingredients that support cell growth, viability, and production while minimizing the accumulation of waste metabolites such as lactate and ammonia.
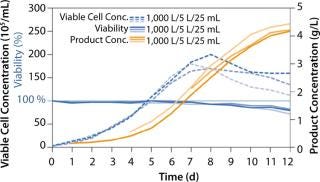
Figure 1: ()
Performance of the ActiCHO Media System
The ActiCHO Media System has been successfully tested with a number of different protein products at different scales. To further demonstrate scalability of the process with ActiCHO Media System in a controlled study, we carried out the same fed-batch process in shake flasks and 5-L and 1,000-L manufacturing-scale bioreactors, with the same feeding regimens from the beginning until the end of the process. We used 25-mL shake flasks, a glass 5-L bioreactor, and an Xcellerex 1,000-L bioreactor. High yields were achieved in all cases, and overall, the results demonstrated scalability and very similar cell growth, viability, and monoclonal antibody production levels with one medium with the same standardized upstream process conditions. Developed by Cellca and PAA, and offered by PAA/GE Healthcare at scale, the ActiCHO Media System, composed of ready-for-use formulations, has proven to be a highly dependable system for rapid, cost-effective process development programs using CHO DG44 cells.
Conclusion
This experiment demonstrates clearly the benefits of using a platform and the ActiCHO platform in particular. The first benefit is the reduction in time and manpower to develop a robust production process that meets all current CMC criteria. It takes six months from DNA to RCB to achieve these results. The second benefit is the reduction of process scale-up risk; knowing that the system will work on large scale in a similar way, resulting in a similar quality product reduces the need for additional process development efforts. This will reduce the CMC development cost for any early phase project. The ActiCHO platform works well with different kind of CHO cells like DG44 and K1.
About the Author
Author Details
Mr. Hugo de Wit and Dr. Abi Abitorabi are the CEO and COO of Cellca GmbH, Germany. Direct inquiries to [email protected].
REFERENCES
1.) Cayli, A. 2011. ActiCHO Media System. BioProcess Int 9:118.
You May Also Like






