Insect Cell Cultivation in Orbitally Shaken Flasks with SensorsInsect Cell Cultivation in Orbitally Shaken Flasks with Sensors
August 1, 2012

Figure 1: ()
The utility of insect cells for protein production has been described in several reviews. Grown in batch mode, production cell lines Sf-9, Sf-21, and BTI-TN-5B1-4 can reach high cell densities (>1 × 107 cells/mL) in an optimized culture medium. Alow-shear cultivation system that guarantees sufficient oxygen supply is also required. The kL a parameter is used to characterize oxygen transfer in a cultivation system.
The 250-mL shaken flasks used in this study were coupled to a Shake Flask Reader (SFR) provided by PreSens. The SFR unit has nine modules enabling wireless real-time monitoring of pH and DO. Using the standard gassing-out method, nine experiments at varying fill volumes (25–150 mL) and shaking frequencies (80–200 rpm) were performed. DO concentrations were monitored over time, and experimental plus model-derived kL a values were calculated (1). Experimental kL a values ranged 4.4–37.9 h−1, corresponding to the modeled oxygen transfer data (data not shown).
Propagations of Sf-9 and Sf-21 cells were performed over seven to eight days in 250-mL shake flasks equipped with integrated and precalibrated pH and DO sensors from PreSens. In each flask, 100-mL medium was inoculated with 5 × 105 cells/mL and incubated at 27 °C and 110 rpm. AkL a of 5.6 h−1 was assumed. In experiment 1, samples were taken from each flask every day. In experiment 2, a sample was taken from only one shake flask each day. Analyses included measurements of living cell density and viability.
The DO course (Figure 1) illustrated the influence of repeated sampling on the flask environment (experiment 1), where a maximum living cell density of 1.6 × 107 cells/mL was reached at day 6 of cultivation. However, no clear statement on mass transfer limitations can be made. The results from experiment 2 (with a noninfluenced DO course) show that cell growth stops at oxygen saturation <10% (1). Based on this and other data, we surmise an oxygen limitation during cultivation day 5, immediately before reaching the maximum living cell density. For all experiments, on-line pH data corresponded well with off-line measurements.
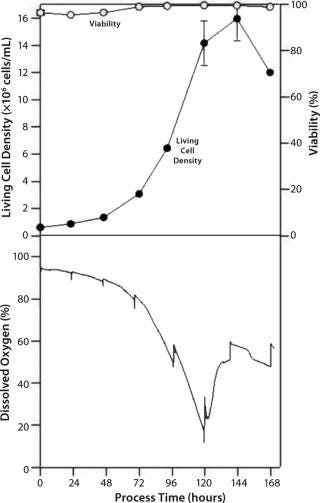
Figure 1: ()
Conclusion
The PreSens’ Shake Flask Reader, which allows noninvasive pH and DO measurement, is advantageous for process characterization and optimized processing of insect cell cultivations at small scale. Oxygen is the key element for high cell density growth in seed train cultivation and protein production.
Note: Applikon Biotechnology partners with PreSens Precision Sensing to provide sensing solutions for bioprocesses ranging <1 mL through >1,000 L.
About the Author
Author Details
Wes Marner is manager of small-scale technologies for Applikon Biotechnology, Inc.; 1180 Chess Drive, Foster City, CA 94404; 1-650-578-1396; [email protected]. This application note is adapted from PreSens application data originally obtained by N. Ries et al.
REFERENCES
1.) Ries, C, G John, C John, R Eibl, and D. Eibl. 2010. A Shaken Disposable Bioreactor System for Controlled Insect Cell Cultivations at Millilitre-Scale. Life Sci. Eng 10:75-79.
You May Also Like






