High-Throughput 96-Well Osmolality Determinations Supporting Cell Culture Process Development and OptimizationHigh-Throughput 96-Well Osmolality Determinations Supporting Cell Culture Process Development and Optimization
July 1, 2009

As the pharmaceutical industry focuses on developing biological drugs derived using cell culture processes, the ability to optimize the growth, health and potential yield of the cell line becomes increasingly important. Finding the optimal cell culture media is a daunting and time-consuming process due to the amount of potential media components and the unique requirements of the cell line being researched. Osmolality information is a crucial component to this research because of its close relationship between cells and media.
Background
Most current osmometers use freezing point depression technology to measure the total solute concentration of a liquid solution. Freezing point is considered the gold standard method in the pharmaceutical industry due to its accuracy and its broad applicability for almost any liquid sample type including complex media mixtures. Historically, high-throughput osmolality determinations have been limited due to the ability of the systems to process only one sample at a time requiring a 2-minute test time per sample. New approaches to cell culture process development and optimization (1) have necessitated faster throughput requirements for osmolality testing. Many pharmaceutical researchers are requiring the ability to increase osmolality sample throughput well beyond the limits of existing osmometers.
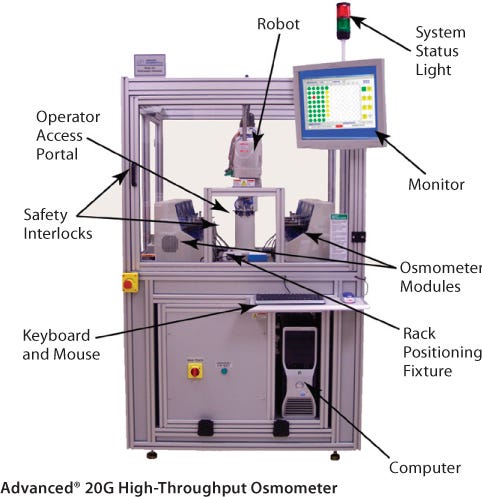
To achieve this goal, a new automated 20G High-Throughput Osmometer has been developed which combines state-of-the art osmometry technology and robotics within a parallel sample processing scheme. Using this system, samples can be analyzed in a 96-well format within 35 minutes with the same accuracy as stand-alone osmometers. The 20G system has been applied to support osmolality testing in even the most demanding cell culture process development schemes in the biopharmaceutical industry.
Functional Specifications
Sample Volume: 20 µL
96-well Format: 12 × 8 layout (compatible with SBS guidelines)
Throughput: 96 samples in 35 minutes
Calibrated Range: 50 to 850 mOsm/kg H2O with ±1% accuracy
User Interface: state-of-the-art touch-screen user interface with simple, intuitive software control
Data Output: exports data as.csv file compatible with all laboratory automation software packages
Regulatory Approvals: certified to applicable safety and EMC standards
REFERENCES
1.) Cheung, L. 2009.Development of an Automated High-Throughput System for Cell Culture Process DevelopmentLab Automation-Automated Cell Cultivation Workshop, Palm:Springs.
You May Also Like






