Cell Therapy BioprocessingCell Therapy Bioprocessing

The past 15 years have seen approval and commercialization of the first cell-based therapeutics, including cartilage repair products; tissue-engineered skin; and the first personalized, cellular immunotherapy for cancer. Those successes are outnumbered, however, by all too common product failures. Notable failures can be attributed to commercial concerns such as high cost of goods (CoGs) and technical hurdles such as inadequate characterization, high process variability, and loss of product efficacy when manufacturing is scaled up (1).
Arguably, the root cause of those commercial and clinical failures is a lack of sophistication in developing living cell-based “drugs” with all the verifiable consistency of any other drug class. Many early cell therapy companies lacked drug development expertise and pursued products that were not feasible commercialization candidates. Some companies, unable to continue with iterative development and further characterization, instead relied on insufficient understanding of their products and ineffective process development, which impaired critical product characteristics and likely set the stage for clinical failure (1).
For cell therapy products to achieve the manufacturing success of biopharmaceuticals, there must be communication and cooperation among three groups of people:
cell processing professionals typically working in academic centers that collectively process hundreds of cell products for transplantation every month
cell biologists who have a strong foundation in product development and cell characterization (as it relates to therapeutic efficacy of cell-based drugs) and can help identify and quantify quality parameters and product specifications
bioprocess engineers with the engineering discipline required to create scalable and robust manufacturing processes (2) that will maintain the critical quality parameters of living cell products while minimizing manufacturing costs inherently expensive products.
Only with a cross-functional approach that encompasses those skill-sets will it be possible to achieve an integrated approach to product and process development that can lead to clinical and commercial success.
The Process and Product Development (PPD) subcommittee of the International Society of Cell Therapy (ISCT) is a group of industry and academic cell therapy professionals aiming to help establish and communicate best practices for integrating the process and product development aspects of cell-based therapeutic development. Its work will include establishing and sharing best practices in establishing specifications for identity, potency, purity, and safety of cell-based therapeutics as well as development of commercially viable bioprocesses to manufacture these products to specifications. These efforts will include driving standardization when possible, sharing best practices within the industry, and helping position early stage, academic-based therapies for potential commercialization.
Cell therapy manufacturing is poised to benefit from know-how and technical innovation that the protein bioprocessing field has driven over the past 20 years (3). Production, storage, and delivery of living cell-based pharmaceuticals presents several unique challenges. Novel, innovative technologies and strategies will be required to bring cell therapies to commercial success.
Major Differences Between Therapeutic cells And Proteins
In just a couple decades, the commercialization of monoclonal antibodies and other therapeutic proteins led to the growth of a multibillion-dollar industry. A great deal of effort has been applied to the establishment of methods for efficient product scale-up and cost reduction of manufacturing processes (4). In addition, a tightly controlled regulatory policy that includes sterility, purity, and quality of raw materials (animal-origin–free, and so on) guidelines has been adopted for manufacturing biotherapeutic molecules. The cell therapy industry has generally been slow to adopt such practices because of specific (sometimes prohibitive) differences between the two product categories.
Recombinant monoclonal antibodies and other therapeutic proteins are generally secreted by genetically engineered cell lines into their culture medium and then can be highly purified, sterilized, and concentrated under well-defined and regulated conditions. By contrast, the product of a cell therapy is the cell itself. That presents a challenge: The end product must be harvested efficiently and cannot be terminally sterilized. Additionally, many cell types currently are not amenable to cell culture systems that exclude animal serum or other animal-origin materials. Further development of serum-free culture systems will be necessary to reach the level of regulatory compliance that is required in the therapeutic protein market.
A major difference between the protein and cell industries is the source of their cells. Many recombinant proteins are produced from selected clones of mammalian cells that are fully characterized, tested, consistent, and stable. Most cell therapies are derived from primary cells isolated directly from tissues that have limited expansion potential. Such cells cannot be selected for optimal performance characteristics, and donor-to-donor variability leads to substantial variability among processes. Scalable production platforms, process validations, and quality control release thus remain as daunting challenges.
Processing Laboratories in Academia
Most clinical cell therapy laboratories at academic medical centers have grown far beyond their origins in processing bone marrow to support blood and marrow transplant programs. They have become powerful resources in development of novel cell-based products. Academic cell processing laboratories commonly serve a range of clinical programs and are closely linked to their academic investigators. Their staffs have developed a valuable breadth of knowledge as well as cutting-edge processing techniques and characterization methods (Photo 1). The most active laboratories produce hundreds (in some cases, thousands) of cell therapy products each year. To date, it is estimated that tens of thousands more “doses” of living cells have been processed in academic laboratories than have been manufactured in all of industry, which represents a remarkable level of experience.
Focusing on phases 1 and 2, such laboratories represent an early stage in the development pipeline. Technology transfer, characterization, and process development activities in an academic processing laboratory thus have great impact on later stages of clinical development and commercialization. It is neither practical nor necessary for an academic cell therapy laboratory to develop commercial-scale manufacturing or testing. It is, however, essential that early stage development work in such laboratories enable rather than impede subsequent commercialization.
To give cell therapy products the greatest chance of success, academic cell therapy laboratory staffs must understand the needs of their industry counterparts further al
ong the development pathway. It is always useful to begin with the end in mind, which can be thought of as establishing a target product profile while in early clinical development. Early stage process development should focus on defining and optimizing a reproducible manufacturing process and establishing a characterization profile that can support product release and subsequent development. Raw materials should be selected and qualified to simplify sourcing and regulatory compliance at later stages of development and commercialization. In the next decade, commercialization of cellular therapies will force most manufacturing from academic laboratories to industry. Leveraging the knowledge of those centers will be important to minimizing wheel reinvention.
Comparing Autologous and Allogeneic Product Manufacture
There are two main manufacturing process paradigms in cellular therapy: patient-specific/autologous and universal-donor/allogeneic products (Figure 1). Historically, most cell therapy products have been patient-specific due to a need for immunologic compatibility. Many cell therapy products in clinical development today, however, are based on cell types that do not give rise to an immune response. Such allogeneic unmatched cell therapies could be more pharmaceutical-like “off-the-shelf ” products.
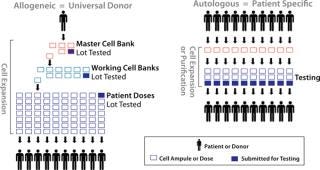
Figure 1: ()
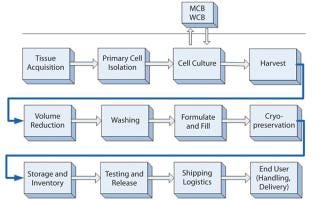
Figure 2: ()
Unmatched allogeneic donor products are amenable to bulk manufacturing, and their production often can take advantage of some aspects of bioprocess technology. Manufacturing commonly involves establishing and qualifying cryopreserved master and working cell banks, then producing large lots of product for release testing. As in biopharmaceutical manufacturing, the process is scaled up during clinical development, and release testing may be performed on lots that represent hundreds or thousands of product doses. The main challenges in allogeneic manufacturing are maintaining a cell product’s critical quality parameters while scaling up its manufacture (stressing cell characterization, as discussed below), and controlling the cost of goods (CoGs) for these inherently expensive products. Although labor and facilities are the greatest component of CoGs for allogeneic cell therapy products during clinical development, it is expected that culture medium and supplements will be the largest part during commercial-scale manufacturing.
Production of autologous, patient-specific cell therapy products are not amenable to a scale-up approach, so their bioprocesses will be scaled out for commercial manufacturing. To achieve efficiencies of scale, high-throughput production by parallel processing of multiple, separate products in automated, functionally closed systems should be widely adopted (5,6). Functionally closed process technology provides product and process isolation, maintaining each product entirely within its own separate, presterilized, disposable processing set. Such systems are highly amenable to automation and associated with extremely low rates of contamination, and they typically improve product yield.
Because each product represents a unique donor, autologous and patient-specific products have the greatest potential for variability, which is a major challenge. Logistics present a challenge as well in that manufacturing each product involves obtaining living cellular raw material. Because patient-specific products are manufactured as one lot per patient, release testing often accounts for the bulk of their CoGs. Despite all these challenges and costs, multiple patient-specific, autologous cell therapy products are on the market or in clinical development today. They generally target applications of major unmet medical need.
Cell Characterization and Potency Analysis
Cell-based products present unique characterization challenges. Producers of small-molecule and recombinant biologic drugs use defined manufacturing and purification processes that yield relatively homogeneous final products with little lot-to-lot variation. Characterization of purity and identity for such drugs can be directly related to several measurable physical traits such as molecular weight, chemical structure, and purity. Drug potency is often correlated with and measured by specific biochemical or cell-based assays in vitro. However, most methods used to characterize the purity and potency of traditional drugs are not suitable for testing cell-based products.
One major challenge to the cell therapy industry is to develop characterization and potency specifications for products with considerable inherent variability — particularly for autologous, patient-specific products. Cell-based products are often derived from donor or patient tissues that contain many cell types. Depending on the stringency of methods used in cell isolation, final products can contain several other cell types in addition to the therapeutic cells of interest. Donor- or patient-based variations in tissue composition add to variability in the final cell product purity and yield. Even products derived from cell lines (e.g., hES or iPS cells) may contain several cell types. Such products require some level of differentiation and expansion, and no current protocol is 100% efficient, so manufacturing produces a mix of undifferentiated or partially differentiated cells in the final cell products.
The most commonly used technology for characterizing cell therapeutics is fluorescence-activated flow cytometry. Multicolor flow analysis allows phenotypic determination of antigens on the surface of a cell. If an appropriate reagent cocktail is used, then both the identity and purity of a final cell product can be determined using a single assay. Gene expression array analysis is increasingly being used, as well. More established analytical technologies such as automated cell counting and enzyme-linked immunosorbent assays (ELISAs) play major roles too. Assay validation, including developing robust reference standards, will have to be addressed as cell therapy products come to market.
Like with traditional drugs, potency assays must be established for each cell-based product before phase 3 clinical trials. However, the mechanism of action for many such drugs is either multifaceted or currently unknown. Developing potency assays for cell products is fraught with ambiguity. FDA guidance suggests including a matrix of assays including gene expression profiles, cytokine secretion, or in vitro cell-based assays showing biological activity such as target cell lysis, inhibition of target cell proliferation, or pluripotent
differentiation potential (7). With the large variety of potential cell products under development, the challenge will be to develop assays that are reliable, reproducible with low variance, inexpensive, simple, and rapid, with appropriate reference standards — that also have relevance to the intended clinical activity.
Common Upstream Processing Platforms
With a wide variety of potential tissue sources (e.g., blood, bone marrow, cord blood, placenta, adipose and other adult tissues, fetal tissue, embryonic stem cell lines, induced pluripotent cells), an important step in the cell therapy manufacturing process is initial isolation or enrichment of a cell population of interest from the tissue source. Several commercial systems are already in use for bulk enrichment and have attributes that facilitate compliance with current good manufacturing practice (CGMP) guidelines. Examples include the CliniMACs system from Miltenyi Biotec (www.miltenyibiotec.com), the Sepax system from BioSafe SA (www.biosafe.ch), and the Elutra system from CaridianBCT Inc. (www.caridianbct.com). For processes that require highly defined cell subpopulations, fluorescence-activated cell sorting (FACS) may have applications, but it presents some complications and limitations (8). Unfortunately, no single cell-sorting instrument platform yet offers a complete, CGMP-compliant system for cell therapy use. However, leaders in the field are pushing forward and adapting current platforms for clinical cell sorting. A challenge for cell product manufacturers will be to find an isolation process that is cost-efficient, easy to use, and CGMP compliant — and that can provide a cell population of the required yield and purity.
Culture processes for expansion and/or differentiation of final cell products (either allogeneic or autologous) are based on current cell culture technology and are ripe for innovations specific to cell therapy application. In general, cells for therapeutic applications are either grown in nonadherent suspension or adherently through traditional two-dimensional (2D) culture. Many cells, such as hematopoietic stem cells (HSCs) and T cells, can be grown in nonadherent suspension culture. That can involve relatively large-scale bioreactors such as bag-based and traditional stirred-tank vessels. Through such means, large numbers of cells can be generated with a relatively small footprint and under tightly controlled conditions.
Other cell types, such as mesenchymal stromal cells (MSCs) (9) from various tissues, are traditionally grown adherently on tissue-culture–treated surfaces. Large-scale expansion of these cell types requires much surface area using traditional 2D methods. Multilayered flasks have been developed to reduce the laboratory footprint per square-centimeter of culture surface. Examples include the CellCube and CellSTACK systems from Corning Inc. (www.corning.com) and Cell Factory system from Nunc A/S (www.nuncbrand.com). But to reach sufficient cell numbers for commercial lot sizes (~1010–1012 cells), large clean rooms (or even whole buildings) would have to be filled with incubators of flasks. Next-generation adherent culture platforms are under development specifically for cell therapy applications, including bioreactors, hyper-density stacked vessels, microcarrier-based cultures, and induced-suspension adaptation growth methods for primary adherent cell types. As the amount of tissue culture surface area per lot increases, the enzymatic harvest and downstream processing of hundreds to thousands of liters of cells will be a new and challenging process bottleneck.
Common Downstream Processing Platforms
Current downstream processing methods for cells use common laboratory equipment for concentrating, washing (clarifying), and formulating them before packaging and storage. Transition to closed, scalable systems will be important to scale-up/out that will be required for commercial production (10). Many culture processes are still at relatively small scale (1- to 10-L harvest), but some midstage allogeneic processes can reach 20–30 L in scale. Cell concentration and washing are currently performed using open centrifugation tubes (≤500 mL) or performed using blood processing equipment.
Recently, bioprocessing technologies such as tangential-flow filtration and continuous or counterflow centrifugation have been adapted for maintaining very high cell viabilities and important biological functions of living products. Cell therapy is benefiting greatly by the move of bioprocess suppliers toward ready-to-use, sterile, single-use systems, but focused development effort specifically for cell therapy applications is greatly needed. The transition to scalable technologies for processing cells will be central to cell therapy success. As processes scale up to 100-L and even 1,000-L harvests, open centrifugation and blood processing equipment will be unable to accommodate such volumes.
Fill and finish processing of therapeutic cell formulations is also in need of standardization. Patient-specific processes typically involve small volumes that are manipulated in biological safety cabinets or through closed bags, tubing sets, and sterile welders. Automation of such processes (5,6) — including automated logistics and tracking — will ultimately be required because single facilities will be processing, releasing, and shipping hundreds of lots per day. Most allogeneic and autologous products are still being filled in blood bags, which are appropriate for small lot sizes. However, as allogeneics increase to hundreds or thousands of doses per lot, a switch to pharmaceutical vials and off-the-shelf filling lines is anticipated (10,11). Large-scale cryopreservation and cold-chain management and logistics will be an engineering challenge as the industry moves forward.

Unique Challenges for hESC Processing
Therapeutic application of human embryonic stem cells (hESCs) offers promising opportunities for addressing diseases using unprecedented stategies. This type of allogeneic process comes with unique challenges, however. These cells can proliferate indefinitely in culture and differentiate into lineage-restricted cells of all three primary germ types (ectoderm, mesoderm, and endoderm). That ability of hESCs to proliferate indefinitely allows for large, characterized, CGMP-compliant cell banks of undifferentiated hESCs to be manufactured and tested. Cells from those banks are thawed and expanded to create large quantities of undifferentiated hESCs, from which differentiation cultures are initiated.
Differentiation culture processes are designed to send hESCs down specific differentiation pathways through chemical signals from their culture microenvironment (e.g., soluble signals in their media or insoluble signaling from an adhesion matrix) until a t
herapeutic cell population of interest is produced. During differentiation, the cells typically pass through several developmental stages and require many media changes with different formulations. Because of these complex processes, differentiation protocols are only now being developed that are sufficiently robust and reproducible to support therapeutics manufacture.
Currently hESCs are cultured in 2D adherent systems on animal-derived surfaces. Unlike many adherent cultures, they are grown as colonies of tightly clustered cells (Photo 2). Cell–cell adhesion is important in chromosome stability, and cells are in many cases passaged as colonies using mechanical methods to remove them from surfaces. The passaging technique limits expansion in multilayered vessels.
Technology development in the emerging field of hESC bioprocessing has focused on addressing technical and raw-material challenges. For example, several defined surfaces including peptides and purified recombinant proteins have been identified recently that support undifferentiated expansion and differentiation of hESCs (12,13,14,15), which marks an important step toward animal-origin–free cultures. Nonmechanical methods for harvesting such cultures from flasks, while maintaining the hESCs in colony form, will make multilayer vessel expansion a possibility. Proof-of-concept work has used suspension culture on either microcarriers or as cell aggregates in suspension, which would enable scalable bioreactor production (2). Establishing comparability of such scaled processes will be critical, which emphasizes the importance of analytical methods for cell characterization and potency. Because hESC production remains at relatively small scales, little work has been performed on scalable downstream processing systems for their postculture manipulation. Cell separation technologies for reducing cellular impurities in final products may need to be developed and scaled, as well.
The Future of Cell Therapy Bioprocessing
Two decades ago, biotherapeutic yields were beginning to reach 100 mg/L, and today’s 10 g/L yields would have been almost unimaginable. Cell therapy bioprocessing is still in its early stages, and innovation focused on addressing specific challenges will bring similar advances. To succeed, commercial success of at least a few late-stage products currently in development will be needed to fund development of next-generation tools and technologies for this field.
The Process and Product Development subcommittee of ISCT is working within the precompetitive space of this field; driving home the importance of integrating product and process development; and stressing the importance of cell characterization, process reproducibility, and standardization. Our end goal is a sustainable industry based on robust, scalable processes that are developed to maintain the critical quality parameters and important biological functions of this new class of living-cell products to address many diseases that currently are untreatable, all at reasonable manufacturing costs (3).
About the Author
Author Details
Ralph Brandenberger is director of process sciences at Geron Corporation; Scott Burger is principal at Advanced Cell and Gene Therapy, LLC; Andrew Campbell is a senior staff scientist for Life Technologies; Tim Fong is technical director for cell therapy at BD Biosciences; Erika Lapinskas is in business development at Sartorius-Stedim; and corresponding author Jon A. Rowley is director of cell therapy research and process development services at Lonza Walkersville, Inc., 8830 Biggs Ford Road, Walkersville, MD 21793-0127; 1-301-898-7025 x2620; [email protected]; www.lonza.com. All are members of the Process and Product Development subcommittee of ISCT.
REFERENCES
1.) Burger, SR. 2010.Manufacturing Cell Therapy Products: Models, Methods, and Process DevelopmentCell Therapy Manufacturing: Stem Cell and Immunotherapies, Informa Life Sciences, London.
2.) Kirouac, D, and P. Zandstra. 2008. The Systematic Production of Cells for Cell Therapies. Cell Stem Cell:369-381.
3.) Mason, C, and M. Hoare. 2006. Regenerative Medicine Bioprocessing: The Need to Learn from the Experience of Other Fields. Regen. Med. 1:615-623.
4.) Birch, JR, and AJ. Racher. 2006. Antibody Production. Adv. Drug Del. Rev. 58:671-685.
5.) Fitzpatrik, I. 2008. Cellular Therapy Success Through Integrated Automation. BioProcess Int. 6:S32-S37.
6.) Hampson, B, JA Rowley, and N. Venturi. 2008. Manufacturing Patient-Specific Cell Therapy Products. BioProcess Int. 6:60-72.
7.) CBER October 2008. Draft Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products, US Food and Drug Administration, Rockville.
8.) McIntyre, C, B Flyg, and TC. Fong. 2010. Fluorescence-Activated Cell Sorting for CGMP Processing of Therapeutic Cells. BioProcess Int. 8:44-53.
9.) Dominici, M. 2006. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells: The International Society for Cellular Therapy Position Statement. Cytother. 8:315-317.
10.) Rowley, JA. 2010. Developing Cell Therapy Biomanufacturing Processes. Chem. Eng. Progr (SBE Stem Cell Engineering Supplement):50-55.
11.) Woods, EJ. 2010. Container System for Enabling Commercial Production of Cryopreserved Cell Therapy Products. Regen. Med. 5:659-667.
12.) Melkoumian, Z. 2010. Synthetic Peptide-Acrylate Surfaces for Long-Term Self-Renewal and Cardiomyocyte Differentiation of Human Embryonic Stem Cells. Nat. Biotechnol. 28:606-610.
13.) Rodin, S. 2010. Long-Term Self-Renewal of Human Pluripotent Stem Cells on Human Recombinant Laminin-511. Nat. Biotechnol. 28:611-615.
14.) Villa-Diaz, LG. 2010. Synthetic Polymer Coatings for Long-Term Growth of Human Embryonic Stem Cells. Nat. Biotechnol. 28:581-583.
15.) Klim, JR. 2010. A Defined Glycosaminoglycan-Binding Substratum for Human Pluripotent Stem Cells. Nat. Meth. 7:989-994.
You May Also Like






