Opportunities in Regenerative MedicineOpportunities in Regenerative Medicine
March 1, 2011
Capitol Hill fly-in days (see the last page of this issue) … A focus of Google Ventures (www.google.com/ventures) … A favored new investment arena for GE’s CEO Jeffrey Immelt, the recently named head of President Obama’s economic recovery advisory panel, and Life Technologies’ Greg Lucier … Hardly a day skipped without a major news publication covering some exciting aspect of the science … The provocative cover of Wired magazine’s (www.wired.com/magazine) November 2010 issue …
It all sounds like the stuff of a major blockbuster industry, but that was regenerative medicine (RM) in 2010. Some people may see this as unfounded hype very divorced from commercial reality; others view it as warranted attention. The general consensus is that a large gap remains between the commercial promise and reality of the cell therapy sector of regenerative medicine. Given the paucity of revenue, rarity of profitability, relative scarcity of venture capitalists interested in early stage companies, and a general feeling within the industry that we’re still waiting for our first tranche of real success stories, most believe that the RM industry is still truly nascent. It is probably not quite as nascent as many might perceive, but it’s nowhere near the powerhouse others might understandably sense given all the buzz. Here I present some metrics on which you can base your own judgment.
Definitions
Here I largely focus on cell therapies, which I will simply define as therapeutics that involve and include “live cells” but not basic, hospital-based, stem cell transplantation. Cell therapy represents several existing and potential business models with as yet little industry precedent or consensus. They are, however, easy enough to understand conceptually. Focusing strictly on the therapeutic side of the industry (rather than companies offering devices, tools, ancillary services, and so on), the business models include
companies that look a lot like traditional biopharmaceutical organizations
others based more on clinical services than has heretofore been the case for biopharma
and still others that might look a lot more like medical device companies — although they are bringing innovative therapies to market.
Cell therapy fits within the large and even more diverse RM industry. RM is typically described as having four primary pillars: cells, devices, biomaterials, and bioactive agents (reagents, drugs, and biologics). Their common goal is to replace, repair, or regenerate human cells, tissues, or organs in such a way as to restore or establish normal functions. If cell therapy is about delivering cells as therapeutics, I often refer to RM as being about affecting cells (including whole tissues or organs) whether through their delivery (cell therapy) or their in vivo recruitment and/or manipulation through molecular or other means. Some Industry Metrics
Regenerative medicine is already commercially available and entrenched into several types of American and Western European clinical practice: tissue engineering (e.g., skin repair/wound healing), orthopedics (e.g., spinal disc and joint cartilage repair), diabetes (e.g., islet cell transplantation), oncology/hematology (e.g., stem cell transplants), ophthalmics (e.g., limbal stem cell deficiency, corneal disease, and so on), and cosmetic/aesthetic (e.g. body sculpting). Cell Therapy Group tracks products that are commercially available and in the development pipeline (under clinical investigation or the subject of late-stage preclinical development). Our industry metrics are limited to industry-sponsored trials and may not accurately capture products in early stage trials, where industry “sponsorship” is less than transparent.
What follows is based on CTG’s data. For another perspective, you can view the presentations given at a January 2011 regenerative medicine briefing hosted by the Alliance for Regenerative Medicine at the BioTech Showcase during a JP Morgan conference (www.alliancerm.org/presentations.html).
Products: Some 275 therapeutic companies with about 240 cell-based therapies are currently on the market or in some stage of clinical development. These therapies can be roughly broken down as follows: ~77 in phase 1, ~89 in phase 2, ~27 in phase 3, and ~44 are commercial (marketed in at least one country).
On the books, we are tracking what we believe is a fairly accurate total of 27 phase 3 or pivotal cell therapy industry-sponsored products in clinical trials: 15 autologous (58%), three allogeneic, three allogeneic–autologous combinations, three allogeneics with devices, one allogeneic with gene modification, one allogeneic–autologous combination with a drug, and one autologous with gene modification. About half those products are fresh in their final formulation (predominately the autologous ones). Seventeen of the 27 are being developed by companies based in the United States, with the remainder in Europe and Asia.
When you scrutinize that list for typical signs of commercial life, only 10 of those trials can be considered “active” by any measure. These can be broken down as follows: six autologous (58–60%), two allogeneic, one allogeneic with gene modification, and one allogeneic–autologous combination with a drug. Six of these are being developed by companies based in the United States.
Industry-sponsored cell therapies in phase 3 pivotal trials are almost 1:2 allogeneic over autologous products. Industry-sponsored cell therapies in all phases of clinical development are roughly 1:1.5 allogeneic over autologous products. Our early analysis of late-stage preclinical studies appears to suggest that the ratio is reversed (~1.4:1), allogeneic over autologous.
Only 30–35% of currently marketed therapies (~13–16) have required and received regulatory approval. By contrast, we estimate that ~90% of therapies in development are “products” that will require premarket approval. Although ~70% of therapies currently marketed were not required to obtain regulatory approval when they were brought to market (and may still not now), this is not a statistical trend expected to continue. It is an artifact of a commercial environment that existed in a relative regulatory vacuum for these types of therapies — certainly a different regulatory framework from what now exists.
For instance, marketed cell therapies under EMA jurisdiction are now in a transitional status. With the exception of the ChondroCelect cartilage repair product from Belgian company TiGenix NV (www.tigenix.com), none of these received EMEA approval under the new advanced therapy techniques and products (ATMPs) regulatory framework. Even previously state-approved cell therapies that are currently available commercially will have to be confirmed either as falling within the nonmedicinal classification (allowing them to fall outside the new regulation requiring marketing approval) or be transitioned under the ATMP regulatory framework. The la
tter products will have to comply with ATMP regulations no later than 30 December 2011 or 2012 (determined by an applicable ATMP category). Market approval applications (MAAs) with supporting scientific, clinical, and manufacturing data will have to be submitted for those to continue on the market.
To date, the EMA has reviewed seven ATMP MAAs thus far. One was approved (the ChondroCelect cartilage product by Tigenix, www.tigenix.com), and another was still under evaluation as of December 2010. I suspect the latter is the Glybera gene therapy for lipoprotein lipase deficiency by Amsterdam Molecular Therapeutics (www.amtbiopharma.com). The others were rejected or withdrawn. I believe that Ark Therapeutics’ (www.arktherapeutics.com) Cerepro gene therapy would be one of those rejected but have no data or speculation as to the others rejected or withdrawn. Some have speculated that one of those might have been Cellerix (www.cellerix.com) beginning to file but then withdrawing for Ontaril cell therapy, which was in a phase 3 trial to treat complex perianal fistulas not associated with Crohn’s disease. But we have seen no data to confirm or deny that speculation.
SKIN REPAIR CELL THERAPIES ON THE MARKET
Apligraf (Organogenesis, Inc.)
Bioseed-S (BioTissue Technologies, GmbH)
CellSpray (Clinical Cell Culture)
Dermagraft (Advanced Biohealing, Inc)
EPIBASE (Laboratories Genevrier)
Epicel (Genzyme Biosurgery)
EpiDex (Modex Therapeutiques)
Hyalograft 3D (Fidia Avdanced Biopolymers
J-TEC artificial skin ChondroCelect (TiGenix)
Laserskin (Fidia Advanced Biopolymers)MySkin (York Pharma)
OrCel (Ortec International, Inc.)
PolyActive (HC Implants, BV)
TissueTech Autograft System: Laserskin and Hyalograft 3D (Fidia Advanced Biopolymers)
About 80% (or ~35) of the nearly four dozen marketed cell therapies worldwide fall into one of two categories: cartilage and skin (wound, ulcer, and burn) repair. The “Skin Repair” box lists marketed skin repair cell therapies, for example. Here are the cell therapies that are marketed, sold, and provided in the United States:
A 2001 premarket approval application (PMA) for the Dermagraft cryopreserved human fibroblast-derived dermal substitute from Advanced Biohealing, Inc. (www.abh.com) was approved by the US FDA’s Center for Devices and Radiological Health (CDRH).
Bio-Tissue, Inc. (www.biotissue.com) filed a 2003 510(k) premarket notification of intent to market for its Prokera device based on cryopreserved amniotic membrane that was approved by the CDRH as a class II medical device.
Although the originator company Forticell Bioscience (www.forticellbioscience.com) is recently bankrupt, its 2001 PMA for OrCel bilayered cellular matrix was approved by the US FDA CDRH.
Genzyme Corporation’s (www.genzyme.com) 2007 humanitarian device exemption (HDE) application for Epicel cultured epidermal autografts was approved by US FDA CDRH. The company’s 2007 biologics license application (BLA) amendment for its Carticel knee cartilage treatment was approved by US FDA’s Center for Biologics Evaluation and Research (CBER).
Organogenesis, Inc. had a 2000 PMA approved by the US FDA CDRH for Apligraf wound-healing treatment.
In 2010, Dendreon Corporation (www.dendreon.com) received BLA approval from CBER for the Provenge autologous cellular immunotherapy.
Some people might include the photopheresis system from Therakos, Inc. (www.therakos.com) in that list because it delivers a cell-based product that is manipulated ex vivo like the stem cell device from Cytori Therapeutics (www.cytoritx.com).
Noncellular RMs on the market include NeuroMend and NeuroMatrix products from Collagen Matrix Inc. (www.collagenmatrix.com); Helitene absorbable fibrillar bovine tendon collagen, NeuraGen and Neurarap collagen nerve guides, TenoGlide and Integra crosslinked collagen-GAG scaffolds, Integra meshed bilayer wound matrix and dermal regeneration templates, and MOZAIK and Integra OS collagen and β-tricalcium phosphate scaffolds, all from Integra Life Sciences (www.integralife.com); Unite stabilized equine decellularized pericardial biomatrix from Pegasus Biologics (www.pegasusbio.com); and AxoGen and AVANCE decellularized human peripheral nerve products from Axogen, Inc. (www.axogeninc.com).
Companies and Revenue: Less than 20% of cell therapy companies are currently public. About a quarter of those with a cell therapy in clinical development have multiple products in clinical development. About 25% of companies are still in the preclinical stage with their lead product.
Most industry analysts agree that the RM market surpassed the $1 billion mark in therapeutic sales in 2007. Table 1 lists the top ten RM products sorted by estimated worldwide revenue that year (1). According to a 2008 report, only two RM products generated more than $100,000,000 in revenue — Infuse by Medtronic and Alloderm by Lifecell — and neither has a cellular component (1). Only one of the top five products in the sector is composed of cells: Genzyme’s Carticel treatment for knee cartilage repair. Although five of the top 10 RM products are cell based, they account for a total of only ~$165,000,000 in annual revenue. Together, they would not qualify as a top 200 drug on the market for 2007.Table 1: Worldwide top 10 regenerative medicine commercial products
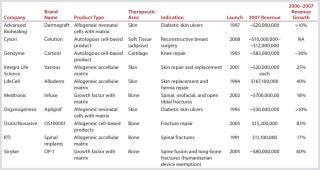
Table 1: Worldwide top 10 regenerative medicine commercial products ()

Based on estimates from the “cumulative numbers of units manufactured and patients treated… as well as from discussions with senior industry experts,” a more recent report values the current RM cell therapy market at ~$100–200 million per year (2). By far the largest contribution to that comes from the Apligraf skin product. In the United States, on average, once every two minutes Monday through Friday, a patient is treated with it (2). And according to Mason and Manzotti, an estimated 675,000 RM cell therapy units have been manufactured as of 31 March 2010, with some 323,000 patients treated by those same products over the same period (2). Observations and Questions
Draw what conclusions you may from the metrics listed here. There are some trends worth noting and some questions worth asking. Pharmaceutical, biopharmaceutical, life science, and healthcare companies are investing heavily in strategy and modestly/cautiously with their money in regenerative medicine. However, with a few
exceptions (e.g., Genzyme and Celgene Corporation, www.celgene.com), biotherapeutics companies are notably absent in these ventures. Governments and nongovernmental organization are investing heavily. Strategic investors (e.g., Google Ventures) have begun participating in the sector. And disciplines and companies outside the industry are beginning to make larger impacts on regenerative medicine: e.g., process engineers, mechanical engineers, manufacturing technologies, genomics, imaging technology, cosmetics/aesthetics, and so on. Despite evidence to date that cell therapies are not (compared with predecessor biotherapeutics) cheaper or faster to develop, do not fail less in development, and have yet to produce profound clinical efficacy over standard care, their promise to cure conditions we currently treat only with palliative care continues to engender great enthusiasm.
Will our institutional, educational, and commercial paradigms allow cell therapy and regenerative medicine to take advantage of the different world in which they are being developed to advance these therapies differently than their predecessors? This is a world of personal genomics (the $100 genome is on its way), bioinformatics, personal connectivity and social media, adaptive clinical trial designs, personalized medicine (diagnostics, biomarkers, theranostics), healthcare reform, aging demographics, increased heal insurance pressure, and workplace virtualization obviating much need for physical infrastructure. Can companies afford not to adapt traditional biotherapeutic development paradigms, given the increasing financial pressures they face and constraints on the old blockbuster model? How will medical tourism and insurance incentives to drive treatments off-shore affect the industry? And what will be the impacts of patient advocacy, expectations, and sophistication over the short and long term?
CELLULAR XENOTRANSPLANTATION
Xenotransplantation applies living cells, tissues, or organs from one species to another, most familiarly from pigs to humans. Before introduction of recombinant proteins in the 1980s, porcine-derived insulin was the most common treatment for diabetics. Researchers have extrapolated a cell therapy based on the idea of implanting porcine pancreatic cells that make insulin (www.dailymail.co.uk/sciencetech/article-1201672/Experimental-diabetes-treatment-inject-humans-pig-cells.html). The concept of cellular xenotransplantation faces many challenges, including the potential for zoonotic disease transmission or endogenous retroviruses and the fact that most animals have shorter lifespans than humans, so their tissues age at a different rate. Currently there aren’t many published cases of success beyond preclinical reports.
Perhaps the greatest obstacle faced by any company choosing to invest in cellular xenotransplantation would be public perception. Even when cells are genetically engineered to prevent immune-system rejection issues, and even if concerns over zoonoses and aging were addressed, the very idea of animal cells used in this way might be intolerable to most people. And some 20th-century quackery may have set the wrong stage, as well.
Dangerous Dealings: The first recorded attempt at “cellular therapy” occurred in 1912 when German physicians attempted to treat hypothyroid children with thyroid cells. An American physician named John R. Brinkley began in 1917 to implant men with tissue from the testicles of young goats, saying it would help with impotence/infertility and cure a wide range of ailments. Ultimately Brinkley’s license to practice medicine was revoked. In the 1930s, Dr. Paul Niehans, called by some “the father of cell therapy” began offering “rejuvenation” therapy (injecting animal cell suspensions) to those who could afford it at his expensive clinic in Switzerland (www.quackwatch.com/01QuackeryRelatedTopics/Cancer/cellular.html). That clinic and others are still in business today.
In 1970 Wolfram Kühnau, an associate of Niehans, began using similar methods to treat cancer patients in Tijuana, Mexico. He claims to have helped those with Down’s syndrome, Alzheimer’s disease, epilepsy, and HIV–AIDS, among other conditions. And now a naturopath named James L. Wilson is promoting products made from bovine mesenchymal cells. He says they can “migrate to any tissue in need of repair.” Wilson even administers the cells under his patients’ tongues — never mind that the digestive system’s primary function is to break down cells, tissues, and proteins into their usable components, not letting them “migrate” intact through the body. There is little discussion, as well, about human immune system’s identification and elimination of foreign bodies such as cells from other species injected into the bloodstream.
And then there’s “sicca cell treatment, also known as cell, dry cell, or fetal cell therapy” (www.ds-health.com/cell.htm). First given to children with mental retardation in the 1950s, the product consists of lyophilized cells from the organs of fetal cattle and sheep. These animal cells are also said to magically migrate to “where they are needed.” The potential adverse effects of such injections are numerous: from anaphylaxis to prion disease.
The public should not confuse valid cellular therapies with all that nonsense. Unfortunately, however, a lay person searching the Internet for information about cellular therapies (whether xeno- or allo- in nature) is just as likely to come across claims of the above as they to find data from the latest GMP-backed clinical trial or press release about an FDA approval. Charlatans like to adopt scientific-sounding language, but instead of regulatory compliance, they back up their claims with conspiracy theories portraying themselves as victims of a medical industry smear job. That makes it hard to counter their claims without seeming like just the bullies they’re talking about. It’s a fine line to walk, but legitimate companies must take steps to separate themselves from purveyors of modern-day snake-oils.
—Cheryl Scott, senior technical editorA Confluence of Evidence
Regenerative medicine and cell-based therapies continue to engender bold prognostications about how they could revolutionize healthcare. These predictions are increasingly coming from people outside the sector, and they are increasingly betting their corporate strategies on the promise coming true. I believe this is based not only scientific data, but also progress on the clinical, commercial, and manufacturing fronts. Slowly this is all enhancing the confidence of skeptics regarding the eventual clinical and commercial viability of this entirely new class of medicines. I hope this brief snapshot of the industry’s current status helps you better understand the industry and assess your own strategies regarding the regenerative medicine sector.
About the Author
Author Details
R. Lee Buckler is founder and managing director of Cell Therapy Group and RegenerativeMedicineJobs.com, as well as a BPI editorial advisor; Suite 417, 103 East Holly Street, Bellingham, WA; 1-778-278-6311; [email protected], www.celltherapygroup.com.
REFERENCES
1.) Smith, DM. 2008.Successful Business Models for Cell-Based Therapies 2008 World Stem Cell Report, Genetics Policy Institute, Wellington:158-162.
2.) Mason, C, and E. Manzotti. 2010. Regenerative Medicine Cell Therapies: Numbers of Units Manufactured and Patients Treated Between 1998 and 2010. Regen Med. 5:307-313.3.) Buckler, RL, R Margolin, and SA. Haecker Prescott, C and J. 2011.Chapter 15: State of the Global Regenerative Medicine IndustryThe Delivery of Regenerative Medicines and Their Impact on Heathcare, CRC Press (Taylor & Francis Group), Boca Raton.4.) Mason, C, and P. Dunnill. 2008. The Strong Financial Case for Regenerative Medicine and the Regen Industry. Regen. Med. www.chrismason.com/general_publications/assets/Mason_Dunnill_Financial.pdf 3:351-363.5.) Mason, C, and P. Dunnill. 2008. A Brief Definition of Regenerative Medicine. Reg. Med. www.chrismason.com/general_publications/assets/Mason_Dunnill_Definition.pdf 3:1-5.6.) PAS 84:2008 2008.BSI Regenerative Medicine Glossary of Terms, BSI, London.7.) Mason, C. 2007. Regenerative Medicine 2.0. Regen. Med. http://celltherapygroup.com/uploads/RegenMed_2.0.pdf 2:11-18.
You May Also Like






