Stem-Cell–Based TherapiesStem-Cell–Based Therapies
March 1, 2011
A recent review of therapeutics in clinical development revealed 68 stem cell-based approaches (1). The majority of those leverage a patient’s own hematopoietic stem cells; others are exploring use of mesenchymal, neural, or embryonic stem cells. Here I highlight new therapeutic applications of stem cells and explore advances in the areas of induced pluripotent stem cells (iPS cells) and process-scale production of stem cells. Both should create new opportunities for stem cell-based therapies.
Types of Stem Cells
Hematopoietic stem cell transplants are routinely given to patients with cancers and other disorders of the blood and immune systems. Autologous transplantation can successfully reconstitute a patient’s own bone marrow and immune system after high-dose chemotherapy (as described in the “50 Years” box). Allogeneic transplantation is used to replace a patient’s defective marrow or immune system.
Current trials are further exploring uses of hematopoetic stem cells for immune modulation, tissue regeneration, and treatment of hematologic cancers. For example, a recent study at the University of Indiana School of Medicine (www.medicine.iu.edu) used genetically modified hematopoetic stem cells to target melanoma (2). Researchers introduced a T-cell receptor gene cloned from a melanoma patient into the hematopoetic stem cells of mice with metastatic melanoma. The potent antitumor gene led to permanent immune reconfiguration and a complete remission of the cancer. A phase 1 clinical trial using this technique is expected to begin in 2011.
The American Type Culture Collection recently introduced primary adipose-derived mesenchymal stem cell growth solutions: cells accompanied by optimized media for functional biomarker expression; normal morphology; and multipotent, lineage-specific differentiation. ()

Mesenchymal stem cells are multipotent stem cells that can differentiate into a variety of cell types including osteoblasts, chondrocytes, and adipocytes. The properties of these cells make them good candidates for use in for tissue engineering. When transplanted systemically, they can migrate to sites of injury in animals.
Launched in 2005, Nuvasive’s (www.nuvasive.com) Osteocel cellular bone matrix contains mesenchymal stem cells and osteoprogenitor cells. The product is used for the repair, replacement and/or reconstruction of bone defects.
Additional applications of mesenchymal stem cells include cardiovascular repair and treatment of ischemic stroke (3,4). A number of studies have made use of mesenchymal stem cells in treating acute myocardial infarction with a patient’s own cells injected directly into damaged cardiac tissue (5).
Cardiac Stem Cells: Recent studies suggest that stem cells may also be able to replace damaged heart muscle cells and establish new blood vessels to supply them. In 2009, doctors at Cedars-Sinai Heart Institute (www.cedars-sinai.edu) in Los Angeles, CA, completed the first such procedure. Stem cells from a patient’s own heart tissue were isolated and injected back into that patient’s heart in an effort to repair and regrow muscle damaged by a heart attack. In the future, stem cells may be dosed along with small molecules to improve their homing abilities and engraftment.
Neural stem cells are self-renewing, multipotent cells that generate the main nervous system phenotypes. These cells are being evaluated for the treatment of highly aggressive glioblastomas at City of Hope Medical Center (www.cityof hope.org), also in Los Angeles. In late 2010, doctors injected neural stem cells with a special enzyme directly into the brain of a patient. The stem cells seek out and attach themselves to cancerous cells. The patient then takes a pill containing a nontoxic drug that enters his or her brain. By interacting with the enzyme in those stem cells, the drug creates an active chemotherapy.
The use of human embryonic stem cells for therapeutic purposes has taken hold more slowly. It was only in October 2010 that doctors began the first tests in patients, injecting stem cell–derived oligodendrocytes into a paralyzed man (6). The study had been initiated in 2009 but was halted for seven months after safety concerns arose from animal studies.
With their potential to yield all possible cell types, embryonic stem cells offer tremendous potential for therapeutic applications. However, safety, ethical, and regulatory concerns — as well as challenges in manufacturing large quantities of these cells — have tempered enthusiasm for them in the commercial sector. Applications using stem cells from a variety of sources will continue to evolve (Table 1). In parallel, new opportunities are arising as a result of our ability to create stem cells from differentiated adult cells.Table 1: Progression of stem-cell–based therapies
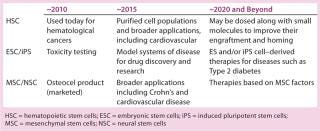
Table 1: Progression of stem-cell–based therapies ()

Induced Pluripotent Stem Cells
Reprogramming adult somatic cells into iPS cells has created tremendous interest since they were first generated from mouse and human fibroblasts several years ago (7,8). These remarkable cells are similar to embryonic stem cells in their ability to differentiate into a wide range of cell types, and they are now routinely generated from adult cells.
Fibroblasts derived from a simple skin biopsy are a common starting point. For example, a researcher can obtain fibroblasts from a patient with Alzheimer’s disease or amyotrophic lateral sclerosis (ALS) and reprogram them into iPS cells. Those can then be induced to differentiate into neurons and other cell types that might be affected in the disease.
The ability to revert somatic cells to an embryonic state and subsequently differentiate them into a range of cell types offers a wealth of opportunities for personalized regenerative medicine and disease research. Because iPS cells can be generated from individuals with different clinical phenotypes and genotypes, they offer a strategic advantage over embryonic stem cells for use in patient-specific cell replacement therapies.
A number of obstacles first must be overcome before iPS cells can be used for therapeutic purposes. Viral vectors and transcription factors must be removed from cells undergoing manipulation. More robust and consistent methods are needed to direct an
d control differentiation into the desired cell types.
Initial efforts to generate iPS cells required simultaneous coinfection of cells with four separate retroviral expression vectors. Each vector carried one transcription factor, which led to a high number of genomic integrations. Alternative approaches include use of plasmids and nonintegrating adenovirus vectors to deliver the transcription factors. However, the reprogramming efficiency (the rate at which cells convert to pluripotency) of the latter is far lower than that of the former (9).
Generation of human and mouse iPS cells now can be accomplished using a single, excisable polycistronic lentiviral vector that delivers all four “Yamanaka” transcription factors: the STEMCCA lentivirus reprogramming kit from EMD Millipore. Use of a single vector significantly reduces the number of viral integrations required. In some cases, iPS clones with only a single viral integrant can be isolated (10). Most recent efforts to reprogram human somatic cells to iPS cells involve synthetic mRNAs encoding the four Yamanaka factors, which eliminates problems associated with genomic integration and insertional mutagenesis (11).
A second barrier to realizing the full potential of iPS cells is the need for robust in vitro protocols for directing their differentiation into cell types of interest. Identifying the right cocktail of media conditions, supplements, and growth factors that successfully and reproducibly drive Ips cells toward a desired lineage is a time-consuming, iterative exercise. A carefully choreographed series of signals must be recreated to guide cells down a chosen pathway. This labor-intensive work has already been performed for a number of cell types. Kits and media containing an optimized set of factors necessary to differentiate stem cells to a chosen lineage are commercially available for generating neurons, oligodendrocytes, mesenchymal cells and osteocytes.
Understanding the microenvironment that iPS derivative cells may face when they are transplanted back into a patient’s body is also critical to the success of cell replacement therapies. Aileen Anderson and her team at the University of California at Irvine (www.anatomy.uci.edu/anderson.html) are exploring whether neurons derived from fetal neural stem cells, embryonic stem cells, and iPS cells can be used to mediate repair in spinal cord injuries.
“Our focus right now is to understand what the role of the inflammatory microenvironment will be in dictating how a cell population responds after transplantation,” she describes. “We’re studying how cells from these different populations are going to be influenced after transplantation in terms of their fate, their migration, and how the environment they see is going to signal back to those cells.”
Anderson’s laboratory is exploring a range of factors that will affect the ability of a cell transplant to mediate repair including the timing and location of transplant, the impact of different immunosuppressants, and the original source of the cells — whether they are fetal-derived neurons, embryonic stem cells, or iPS cells. Tools for Discovery
Disease-specific iPS-derivative cells are being used for disease modeling and to support small molecule drug discovery and development. Uses include facilitating target discovery, screening lead compounds, and improving toxicity evaluation and metabolic profiling (Figure 1).
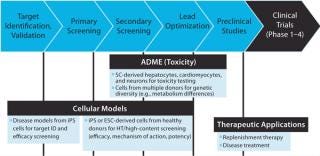
Figure 1: ()

Disease Modeling: For decades, researchers have relied on animal models, immortalized cell lines, or short-lived primary cultures to dissect the mechanisms and pathogenesis of diseases. Genetic manipulations including overexpression, knock-down, knock-out, and knock-in strategies are often used with animal models in an attempt to replicate genetic patterns linked to specific disease phenotypes.
Differentiated cells derived from iPS cells have the potential to transcend the inherent limitations of existing disease model systems. Cells derived from patient-specific iPS cells could provide a more relevant model system. Their properties more closely resemble a patient’s system, and they don’t require genetic manipulation.
“Diseases that arise from single base mutations or deletions are certainly well-suited for modeling with iPS technology,” notes Gustavo Mostoslavsky, codirector of the Boston University Center for Regenerative Medicine (www.bumc.bu.edu/stemcells). “But more complex diseases and those that do not have robust animal models also have the potential to be modeled using iPS cells.”
Mostoslavsky’s laboratory is generating intestinal epithelial cells from iPS cells to study disorders such as irritable bowel and Crohn’s diseases. “Recent studies have shown a role for macrophages in Crohn’s disease,” describes Mostoslavsky. “Another paper showed triggering of the disease by a virus when a particular gene mutation is present in mice” (12).
The lab is developing a human model system of intestinal epithelial cells and macrophages derived from Crohn’s patients by way of iPS cells and will ultimately compare those with the same cell types from disease-free individuals. The virus suspected of playing a role in Crohn’s can then be introduced into both systems.
Chad Cowan’s laboratory at the Harvard Stem Cell Institute (www.hsci.harvard.edu) is using iPS cells to support studies of obesity and metabolic disorders. Although his lab can easily obtain fat cells from patients, those cells can’t be cultured over the long term. “We can keep the fat cells alive for a short period, but that only allows us to do a one-time endpoint assay. It doesn’t allow us to tease out the complexities of what might be going wrong in a patient with a metabolic disorder. The ability to make patient-specific fat cells from iPS cells completely changes the game.”
With iPS cells, the lab can conduct dozens of assays to identify differences in fat cells from a person with a metabolic disorder such as type 2 diabetes versus an unaffected individual. The ability to take a single genotype and potentially make any tissues that might be involved in a metabolic disorder — such as pancreatic beta cells, hepatocytes, or hypothalamus cells — can lead to a powerful disease model.
Drug Screening: Using iPS-derivative cells, potential therapeutics can be screened against a large number of patient-specific cells before initiating clinical trials. Variation in the response to drugs by cells of patients with genetic differences can guide targeted selection of patients for enrollment in clinical trials, resulting in studies that are smaller and more likely to be successful.
James Ellis, senior scientist at the Hospital for Sick Children in Toronto (www.sickkids.ca/Research/Ellis-lab) and scientific codirector of the Ontario Human iPS Cell Facility (www.ontarioips.ca), is interested in drug screening applications for cystic fibrosis. He readily sees the va
lue in the derivation of lung cells from iPS cells.
“Obtaining lung cells from a patient with cystic fibrosis is really only possible when they’ve undergone a lung transplant. But one consequence of the disease is that patients have dramatic lung infections, so it’s very difficult to establish primary cell lines. Even if you did, those will have a limited ability to be passaged. You may not be able to make enough cells to complete or verify your screen.”
Through use of iPS cells, the Ellis laboratory plans to generate large numbers of cells from a range of patients. Genomic patterns can then be cross-referenced to drug screening results. “You can then compare one patient with others and maybe start to make predictions as to which drugs are going to work in which patients.”
Investigative Toxicity: Differentiation and expansion of human iPS cells into functional hepatocytes for use in investigative toxicity studies could overcome the shortcomings of primary hepatocytes and immortalized cell lines. Use of iPS-derived hepatocytes (and other cell types commonly used for toxicity studies) offers a number of important advantages to investigative toxicity studies including
availability of a consistent source of cells that more closely match in vivo phenotype and physiology
elimination of reliance on sporadically available donor sources
reduction in use of animal models and animal tissue
a more standardized, reproducible process for toxicity testing
improved predictive capabilities of early toxicity studies for less frequent late-stage attrition of drugs.
More efficient and predictive toxicity studies enabled by iPS-derived cells can be expected to reduce development costs associated with the late-stage failure of drug candidates. Identifying those candidates with toxicity concerns earlier in discovery can improve the safety — and ultimately the success — of clinical trials. Large-Scale Productction
As demand for stem cells in both drug discovery and therapeutic applications grows, effectively translating their promise into reality will require large-scale “industrialized” production under tightly controlled conditions. Achieving that while meeting rigorous quality and regulatory standards will depend on further progress in cell culture and scale-up, characterization, enrichment, purification, and process control to safely and cost-effectively deliver a consistent and reproducible supply of cells.
50 YEARS OF SUCCESS IN STEM CELL THERAPY
Just as blood transfusions can be considered the first human cell therapies, we can think of bone marrow transplants (used to treat certain cancers and blood disorders) as the first stem cell therapies. Multipotent stem cells in bone marrow give rise to blood cells — replacing cancerous white cells in leukemia patients, for example. A patient’s own bone marrow (and its stem cells) is killed by chemotherapy and/or radiation, then replaced with some from a healthy, matching donor. In successful transplants, injected stem cells migrate to a patient’s bone marrow to begin producing new, healthy blood cells. (Unlike the animal-cell–based quackery described elsewhere in this special issue, these human cells from carefully selected donors really can perform the migration that purveyors of fake cell therapies claim — without so much risk of immune response.)
An early leader in bone marrow transplants — and consequently, stem cell research — is the Fred Hutchison Cancer Research Center (www.fhcrc.org) in Seattle, WA, founded in 1972. Pioneering work of E. Donnall Thomas in bone-marrow transplantation at the center led to a 1990 Nobel Prize in medicine, by which time his once-“radical” ideas had made FHCRC the recognized leader in the field. Early bone-marrow transplants in the 1950s involved only identical twins, and the first successful nontwin sibling procedure was conducted in 1968. An unrelated transplant first succeeded in 1973, and FHCRC was the site of the first such transplant to treat leukemia shortly thereafter.
An NIH-funded FHCRC institutional self-analysis of transplant-patient outcomes reported in fall of 2010 on a decade of refinements in marrow and stem cell transplantation for treating blood cancers at FHCRC. The study compared transplant-patient outcomes in the mid-1990s with those a decade later. Results showed a 60% reduction in the risk of death within 200 days of transplant and a 41% reduction in the risk of overall mortality.
“Everything we looked at improved a decade after the initial analysis,” said George McDonald, MD. He and his colleagues reviewed the outcomes of 1,418 transplant patients who received peripheral-blood stem cells or bone marrow from unrelated donors between 1993 and 1997 and compared those with 1,148 patients who had the same procedures between 2003 and 2007. Malignancies treated included forms of leukemia, lymphoma, multiple myeloma, and myelodysplastic syndrome. Estimated one-year overall survival rates for both groups were 55% and 70%, respectively, with statistically significant declines in the risks of severe graft-versus-host-disease; infections caused by viruses, bacteria, and fungi; and complications caused by damage to the lungs, kidneys, and liver.
Several improvements in clinical practices improved risk and outcomes. For example, use of donor peripheral blood hematopoietic cells instead of bone marrow provided for faster engraftment and return of immunity. Donor matching for unrelated patients had also improved over time.
“This research and the improved outcomes are the result of a team approach to one of the most complex procedures in medicine,” McDonald said in a press release, crediting medical oncologists; transplantation biologists; specialists in infectious disease, pulmonary and critical care medicine, nephrology, gastroenterology, and hepatology; as well as nurses and support staff. “These data show clearly that our collective efforts have improved the chances of long-term survival for our patients.”
The National Bone Marrow Donor Registry was federally funded in 1986, and in 1987 the first donor match was made. In 1988, the name was changed to the National Marrow Donor Registry (NMDP), and it now includes a network of donor registries in 30 countries. The database contains more than 5.5 million donors, facilitating some 200 transplants every month.
—Cheryl Scott, senior technical editor
In both autologous and allogeneic therapies, cells are harvested from donors or the patients themselves, grown to sufficient quantities, and then retuned. Cell replacement therapies derived from embryonic stem cells or iPS cells present a more challenging bioengineering and manufacturing feat. Techniques used by the pharmaceutical industry for manufacturing protein-based drugs must evolve — or in some cases be completely reengineered — to support the manufacture of cell-based therapeutics. I contributed a review of related challenges and advances to the October 2010 issue of BioProcess International (13).
Stem cells represent seemingly limitless clinical applications. Yet along with this great promise come numerous challenges. Taking full advantage of the unique properties of these cells will require advances in our knowledge of their inner workings as well as development of new approaches to their large-scale production.
A VETERINARY SUCCESS STORY
Formed in 2002, Vet-Stem Inc. (www.vet-stem.com) is the first company to offer regenerative medicine to veterinarians (1). In 2003, the company introduced the first veterinary stem cell service in the United States. Its technol
ogy has been used to treat tendon, ligament, and joint injuries in more than 3,000 horses and more than 1,000 dogs. Under cofounders Robert Harman, DVM, MPVM, and Michael Dale, the company holds exclusive worldwide licenses to adipose regenerative cell technology from the University of Pittsburgh (through its licensee, Artecel Inc., www.artecel.com), the University of California, and Hawaiian company Tissue Genesis, Inc. (www.tissuegenesis.com). Vet-Stem’s own pending patents cover uses and methods of procurement and delivery of related cells in veterinary and human medicine.
Adult fat has a high progenitor cell concentration available in quantities that can supply a therapeutic dose without cell culture. That’s why Vet-Stem uses regenerative cells extracted from adipose (fat) tissue, which is readily available and highly metabolic. Adult stem cells used for joint disease and tendon and ligament injuries are taken from an animal’s fat reserves, isolated from surgically removed fat at a company laboratory, then in 48 hours returned to the veterinarian, who injects them into an arthritic joint, tendon, or ligament, where they accelerate and optimize the animal’s innate natural healing process. Adult stem cells produce growth factors and stimulate resident cells to become more active, reduce proinflammatory mediators and increase antiinflammatory mediators in the tissue, and “home in” directly to injured tissue.
“This puts stem cell therapy into the present day instead of a future theoretical concept,” said Harman in a press release, “Now there are new and effective treatment options for injuries that in the past would have ended the career or usefulness of a horse.”
Last fall, the company announced its lifetime donation of stem cell treatments to treat the war injuries of Lex, a canine commemorative Purple Heart recipient who was a Marine Corps bomb-sniffing dog stationed in Fallujah, Iraq. The dog survived a rocket-propelled grenade blast in March 2007 that left him severely injured and took the life of his handler, Corporal Dustin J. Lee. Since that time, Lex has struggled with several problems related to his injuries, including chronic arthritis. His vet, Dr. Lee Morgan, made Vet-Stem aware of the case. About half the dogs treated with stem cell therapy need to be retreated within two years. The company stores more than 18,000 doses of stem cells for thousands of animals and can create more using stem cell culture technology.
“We are pleased to be a part of this great effort and to do our small part in providing comfort to Lex and the Lee family,” said Harman in a press release, “and we appreciate Dr. Lee Morgan’s contribution and discounted services.” Dr. Morgan noted, “This is one of the most important patients I have seen in 14 years of practice. I was surprised to see how quickly Lex responded to the stem cell therapy.”
Reference
1 Young RR. Executive Summary: 2010–2020 Analysis and Market Forecasts. 5th Annual Stem Cell Summit, 16 February 2010, New York, NY.
—Cheryl Scott, senior technical editor
About the Author
Author Details
Robert Shaw is commercial director of the stem cell initiative at Merck Millipore, 290 Concord Road, Billerica, MA 01821; 1-781-533-2511; [email protected].
REFERENCES
1.) McKernan, R, J McNeish, and D. Smith. 2010. Pharma’s Developing Interest in Stem Cells. Cell Stem Cell 6:517-520.
2.) Ha, SP. 2010. Transplantation of Mouse HSCs Genetically Modified to Express a CD4-Restricted TCR Results in Long-Term Immunity that Destroys Tumors and Initiates Spontaneous Autoimmunity. J. Clin. Investigation 120:4273-4288.3.) Psaltis, PJ. 2008. Concise Review: Mesenchymal Stromal Cells: Potential for Cardiovascular Repair. Stem Cells 26:2201-2210.4.) Doeppner, TR, and DM. Hermann. 2010. Mesenchymal Stem Cells in the Treatment of Ischemic Stroke: Progress and Possibilities. Stem Cells Cloning 3:157-163.5.) Martin-Rendon, E. 2008. Autologous Bone Marrow Stem Cells to Treat Acute Myocardial Infarction: A Systematic Review. Euro. Heart J. 29:1807-1818.6.) Strauss, S. 2010. Geron Trial Resumes but Standards for Stem Cell Trials Remain Elusive. Nature Biotechnol. 28:989-899.7.) Takahashi, K, and S. Yamanaka. 2006. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures By Defined Factors. Cell 126:633-676.8.) Takahashi, K. 2007. Induction of Pluripotent Stem Sells from Adult Human Fibroblasts By Defined Factors. Cell 131:834-835.9.) Baker, M. 2008. Integration-Free iPS Cells. Nature Reports Stem Cells.10.) Sommer, CA. 2010. Excision of Reprogramming Transgenes Improves Differentiation Potential of iPS Cells Generated with a Single Excisable Vector. Stem Cells 28:64-74.11.) Warren, L. 2010. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell in press.12.) Cadwell, K. 2010. Virus Plus Susceptibility Gene Interaction Determines Crohn’s Disease Gene Atg16L1 Phenotypes in Intestine. Cell.13.) Shaw, R. 2010. Industrializing Stem Cell Production. BioProcess Int. 8:10-15.
You May Also Like






