Single-Use Technologies in Cell TherapySingle-Use Technologies in Cell Therapy
March 1, 2013
Single-use technologies (SUTs) are tools that can be used in producing cell therapies and personalized medicines. Such products must meet specific requirements because of the way they are used. To meet those criteria, the cell therapy industry simply has no alternatives to single-use systems.
SUT applications are rapidly changing. Traditional uses for single-use systems in cell therapy include processing in clinical settings (e.g., blood bags, transfer sets) and research and development (e.g., T-flasks, pipettes). Although such applications continue, the commercialization of both autologous and allogeneic cell therapies presents new challenges with SUT scale-out and scale-up. Fortunately for those of us working in cell therapy, the biopharmaceutical industry has been actively addressing the effect of SUTs in manufacturing. Resulting studies provide a wealth of supporting data and guidance documents. They have also led to SUT manufacturers having a stronger awareness of such production issues.
SUTs are widely used and accepted in the cell therapy industry. Large-scale production and eventual commercialization, however, will create vastly different end-user requirements for single-use systems. Knowing what testing and validation requirements are needed, when to consider them, and how to avoid pitfalls will help ensure that users have long-term success.
Overview
Single-use (also referred to as disposable) technologies and systems are those intended for one-time use. They generally consist of plastic polymers that include but are not limited to polyethylene (PE), ethylene vinyl acetate (EVA), polyvinyl chloride (PVC), polypropylene (PP), and polycarbonate (PC). Disposables originated in the early 1950s by Fenwal Laboratories (now Fenwal Blood Technologies, a Fresenius Kabi Company) in the form of plastic blood bags. Since that time, SUTs have grown into an extensive range of products: from standard Petri dishes and culture flasks routinely used in cell culture laboratories to large-scale cell stacks and rocker bags (Figure 1) and now even immense 3,000-L culture bags for commercial-scale production levels (1). Although early uses were dedicated to regulated (medical device) blood-transfusion applications, the latest advances can be attributed to wide-scale use and adoption into biopharmaceutical production (2). Such increased use and adoption by the industry has been a driving force for improving overall quality standards for SUTs.
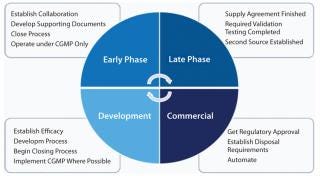
Figure 1: ()
Using disposable technology for cell therapy development, manufacturing, and clinical application is standard practice. Cell therapy experts are very familiar with SUTs. But the hybrid of disciplines and applications making up the industry has in many ways created some confusion when it comes to the next generation of considerably different cell therapies currently in clinical development.
Consider, for instance, a group working on an exciting new therapy originating from a hospital/clinical setting. Such personnel are well versed in working within a clinical environment mainly using regulated (medical device) SUTs. Now consider a completely different therapy being developed by a research laboratory at a university level, which in turn eventually spins off into a new start-up cell therapy company. Those personnel are adept at working in a nonregulated environment with standard R&D labware. As such, the knowledge and perceived requirements for SUTs for clinical scale-up and product manufacturing is likely to be noticeably different. In both cases, small-scale development usingestablished single-use technologies and systems introduced initially will be acceptable. But it’s likely that neither will have appropriate technology and necessary documentation to support manufacturing requirements in later clinical phases.
With more cell therapy products entering phase 3 clinical trials and commercialization, it is imperative that the biopharmaceutical industry recognize not only the opportunities presented with SUTs, but more important, the knowledge gaps and potential pitfalls. It can be devastating to reach a point when a product is ready to enter a phase 3 clinical trial (or, worse, commercialization) and have to make a change to a single-use cell growth/expansion bag or storage container because another technology was discontinued or altered. Given the vast availability of alternative (similar) SUTs, making a change doesn’t seem too daunting a task until you notice, for example, that your cells don’t behave the same way with the new film, or the bags don’t meet the same storage requirements. That situation could significantly delay timelines or even destroy potential product.
Fortunately, manufacturers already have learned from encounters with such issues. Some have incorporated single-use platforms for producing new commercialized drugs. Along with this, the SUT industry suppliers and service providers of the Bio-Process Systems Alliance (BPSA, www.BPSA.org) help establish guidance and best practices for implementing single-use in biomanufacturing processes (2). The cell therapy industry can benefit from using current resources and developing a better understanding of the impacts of disposable systems on manufacturing.
Implementation Considerations
SUTs are available for development, upstream and downstream manufacturing, and delivery of cell therapies. Applications range from simple media storage to complex cell harvesting/processing, cell culture, activation, analysis, final fill, and storage. Advantages of SUTs are well documented (3), but some aspects may not be as apparent when applying single-use to cell therapy manufacturing. You should be familiar with the various SUT components, understand where they come into play, and when and how they affect development. Table 1 lists common items to be considered as they relate to basic material requirements, testing and validation aspects, and regulatory support needs (4).
Table 1: Common requirements and validation support for single-use technologies and systems

Table 1: Common requirements and validation support for single-use technologies and systems ()
Material requirements include gas permeability and glass-transition temperature (standard film qualities associated with SUTs). Such parameters can be essential to material behavior and are often in product datasheets. Validation and regulatory support testing are typically performed on an entire container. The extent to which such tests have been completed varies greatly among products, especially for custom-designed disposables. If testing has been performed, much of the resulting data will be available with product validation guides.
Criteria listed in Table 1 are typical for SUTs provided to the biopharmaceutical industry, but issues such protein adsorption or particulates are not as common to cell therapy. Particulates, for example, are routinely tested in bioprocessing, and standards for their detection and identification exist. However, currently in cell therapy creating a similar standard for particulates is not practical and willrequire more discussion (5). With the personalized nature of many cell therapies, each requirement and test will be unique. Therefore, they should be evaluated individually for their impact on development.
Long-Term Impact Considerations
As cell therapies continue to advance and more products gain approval or commercialization, long-term factors of using SUTs need to be considered. Table 1 lists some topics that should be evaluated to determine the potential impact on each specific product. Although no one wants to think about items such as supply security or scalability at the preclinical level, it’s certainly a good idea to start as soon as possible because waiting until a product is approved is probably too late.
Security of Supply and Secondary Sourcing: Because of the high process variability in cell therapy, customized disposable systems may be needed. But they can also put your processes and your products at high risk, mainly because the product will be single-sourced. Therefore, you should map out a supply chain for critical components and have contingency plans in place for everything from an entire single-use system to individual components and resin materials. Fortunately, technology suppliers have most if not all of that information available when needed. Taking precautions to ensure supply security may not appear to be significantly important during development, but a lot can happen in the long span of time it takes for cell therapy products to gain approval. If a mitigation plans have already been established, then they should be tested.
How do you ensure that a container or film used — and critical to your process — doesn’t change? That is where supply security awareness will be key. Discuss specific items with suppliers to know what plans they have in place. You can then determine whether additional security measures are required that will lead to some level of commitment from each party.
Another widely used risk-mitigation strategy is to investigate availability of secondary sourcing. Following the discontinuation of important products over the past several years, second sourcing has become more the norm these days. It can be accomplished on a product-specific level (through a manufacturer or other source). For some commonly used SUTs, standard products are available from multiple manufacturers (e.g., cryopreservation bags), and basic comparison and validation testing can be performed on them. Challenges arise when customized technology or systems are needed. In such cases, careful planning and attention are imperative. Not all manufacturers are willing to support custom development, but some like Charter Medical specialize in developing and supporting implementation of custom single-use closed systems for cell therapies.
Scalability and Automation: Challenges associated with scalability (up or out) for cell therapies have been well documented. They include difficulties with system closures, processes, and transitions to automation (6,7,8). Each SUT should be evaluated to ensure that major items and systems are scalable. This also becomes important if custom disposable systems are used in manufacturing cell therapies. For example, TxCell (a cell therapy company focused on developing cell-based immunotherapies) required a scalable, custom single-use point of care device. Working closely with the single-use supplier (Charter Medical) helped mitigate scale-up risk and delays (www.pharma-iq.com/manufacturing/podcasts/txcell). You should determine whether a single-use product is amenable to volume changes and whether its manufacturer has the capacity. Often, those items won’t present an issue, but you should spend time with suppliers to make sure everyone is aware of the situation.
Disposal and Regulatory Approvals: The biopharmaceutical industry — in collaboration with single-use manufacturers — is actively addressing SUT disposal issues. A good overview of some of the strategies was prepared by the disposals committee of the BPSA (9). Regulatory approvals (e.g., FDA, CE) are highly application-specific and will be a collaborative path that includes both end-user and SUT manufacturers. Gathering data and information early and often to support SUTs within your process will help you minimize the problems and should prepare you for the long term. Be sure to work closely with single-use manufacturers; they are valuable resources (6).
Ultimately, a specific cell-therapy product, application, and development stage will determine the necessary requirements and validation. Those properties and others come into play at all facets of single-use applications. Some criteria have been adopted from the biopharmaceutical industry, but others will require development (e.g., potency) or adaptation (e.g., particulate) for cell therapy applications.
End-user Feedback
Learning from our peers in the industry about what works and what doesn’t is highly beneficial to future success in the topics discussed above. We do this largely through peer-reviewed publications and through numerous conferences and scientific meetings. But with the complexity (and proprietary nature) of cell therapy — each product and process being unique — we can find ourselves working in a bubble. So it becomes inherently difficult to establish modelsand standards. One of the best ways to better understand how to navigate the minutiae of single-use in cell therapy applications is to learn from end users who are actively pursuing them.
What are some potential pitfalls and areas for improvement when working with SUTs in cell therapy? To investigate, we asked subsets of end users at various stages of development ranging from phase 1 through completion of phase 3 about their experience with SUTs. Questions and the summarized answers are below.
At what stage of the development process is it important to begin gathering the required supporting documents? The consensus was as early as possible (phase 1), and those documents should be in place before starting phase 3.
What are the most important aspects to consider when qualifying SUTs (process specific)? Users identified the following: impact on quality and function (performance) of cellular product, adaptability to process, biocompatibility, leachables and extractables, regulatory status and labeling (not for research use only), an
d qualified vendor and supporting product validation (cGMP, stability, expiration).
When considering scale-up or scale–out, what concerns do you have with SUT? Answers included supply chain stability and the importance of having a second source available; and cost and limitations to automate, especially with critical customized solutions.
For those just starting (development), what are some common pitfalls that can and should be avoided with SUTs? Users’ answers included
Not establishing a collaborative relationship with SUT manufacturer up front
Not obtaining compatible materials that are both scalable and reliable for long term
Not using high-quality (non- CGMP, particulate load) products that lack sufficient supporting validation packages.
Not having supporting information up front.
What can manufacturers and suppliers of SUTs do to support the cell therapy industry? Responses included
Improve data and support (validation, regulatory) packages
Make more options available for small/very small volumes
Establish more options to support custom single-use solution needs
Develop long-term commitments to manufacturing and validation support.
Feedback from end users clearly indicates common pitfalls with SUTs and areas where improvements can be made for cell therapy. Figure 1 shows some of those points. Responses were quite different between those responding from a clinical background and others with a manufacturing interest, however. Even with a limited sampling group, general consistencies were apparent, and extending the study could prove highly beneficial to the cell therapy industry. Although single-use technologies and systems are innovative, they can’t always provide an acceptable and easily adaptable strategy for cell therapy manufacturing. So be aware of the requirements and know when to address them in your system (Figure 1).
Lessons for the Future
Given the vast product requirements and the personalized or custom nature of cell therapies, there are no alternatives to SUTs. Although the applications of SUTs for cell therapy are certainly common, their use for large-scale manufacturing is relatively new. It is important to know and understand the specific requirements of a cell therapy when implementing SUTs and its potential long-term impacts. Waiting until you enter your phase 3 clinical trial to inquire about the scalability of your SUT or security of supply can severely jeopardize success. Recognizing and addressing requirements as early as possible in development is imperative. Clearly, the industry needs new technologies and continual involvement from SUT manufacturers to provide custom solutions and supporting validation. The key will be establishing collaborations between end users and manufacturers; both parties can benefit significantly from working together. Many lessons can be learned about SUTs from other industries, but our peers within cell therapy may be the best source of information moving forward. Just as the biopharmaceutical industry has had a major impact on the development of new SUTs and supporting documents over the past 10 years, so too will the cell therapy industry have a decisive influence on the future.
About the Author
Author Details
Dominic Clarke, PhD, is the cellular therapy and cryogenic storage product manager at Charter Medical, 3948-A Westpoint Blvd., Winston-Salem, NC 27103; 1-336-714-4217; [email protected]; www.Chartermedical.com.
REFERENCES
1.) Kapp, T. 2010. Road Map to Implementation of Single-Use Systems. A BPSA White Paper for Manufacturing Decision-Makers within End-User Organizations. BioProcess Int. 8:S10-S19.
2.) Whitford, WG 2010. Single-Use Systems as Principal Components in Bioproduction. BioProcess Int. 8:34-42.
3.) Ott, KD 2011. Are Single-Use Technologies Changing the Game?. BioProcess Int. 9:S48-S51.
4.) Barbaroux, B, and A Sette. 2006. Properties of Materials Used in Single-Use Flexible Containers: Requirements and Analysis. BioPharm Int.
5.) Clarke, D. 2012. Managing Particulates in Cellular Therapy. Cytotherapy 14:1032-1040.
6.) Clarke, D 2012. Implementing Custom Single-Use Solutions for Cell Therapy Production. BioProcess Int. 10:S32-S35.
7.) Brandenberger, R. 2011. Cell Therapy Bioprocessing: Integrating Process and Product Development for the Next Generation of Biotherapeutics. BioProcess Int. 9:30-37.
8.) James, D 2011. Therapies of Tomorrow Require More Than Factories from the Past. BioProcess Int. 9:S4-S11.
9.) 2007. BPSA Disposals Subcommittee. Guide to Disposal of Single-Use Bioprocess Systems. BioProcess Int. 5:22-28.
You May Also Like






