Glass Delamination and BreakageGlass Delamination and Breakage
Although glass is widely considered to be the most traditional and cost-effective option for a parenteral drug container or delivery system, it may not always be the most economical or the best choice for certain products. With knowledge emerging about the suitability of materials in contact with drug products, it is time to look at alternatives that may offer a more appropriate choice and mitigate the risks associated with glass. As single-use technology finds its way into upstream and downstream processing, might such materials be useful in product containment as well?
PRODUCT FOCUS: PARENTERALS
PROCESS FOCUS: FORMULATION, FILL AND FINISH
WHO SHOULD READ: PRODUCT AND PROCESS DEVELOPMENT, MANUFACTURING
KEYWORDS: Container–closures, leachables, extractables, drug product formulation, contamination
LEVEL: BASIC
Other Product Recalls
Recalls related to glass delamination, particulate, and breakage issues have spurred the need for greater understanding of risks associated with materials used in container–closure systems. Product-contact materials qualified for end use should not affect a drug’s manufacturing, quality, safety, or efficacy. Unanticipated events related to packaging drug products can lead to shortages and financial losses. Over the past five years, more than 20 product recalls have been associated with glass issues, and over 100 million units of drugs packaged in vials or syringes have been withdrawn from the market (1). Each recall can prove costly not only to a manufacturer’s bottom line — through investigations, loss of market share, and replacement costs — but also to its reputation in the marketplace. Patients can suffer as well if their only source of therapy is cut off even temporarily.
In 2011, American Regent recalled its USP-grade calcium gluconate injection because of silicone particulates found in glass vials of the product. The company also recalled USP-concentrated sodium chloride and methyldopate hydrochloride injection products because of possible contamination with particulates when some vials exhibited translucent visible particles consistent with glass delamination (2,3,4). The “Other Product Recalls” box lists some instances involving other companies.
Although no reported incidents have been directly associated with adverse effects caused by glass, the presence of glass flakes could pose considerable risk to patients. When injected into humans, the flakes can cause embolic, thrombotic, and other vascular events. In addition, such contaminants could cause development of subcutaneous foreign-body granulomas as well as local injection-site reactions and increased immunogenicity. Based on the number of recalls lately related to glass delamination, the US Food and Drug Administration has advised drug manufacturers to “re-examine their supplier quality management program with glass vials manufacturers to assure that this phenomenon is not occurring” (5).
Other Product Recalls
Additional recalls related to glass include
Hexal Pharmaceuticals: 5-fu Hexal (glass particles in vials) in October 2010
Ebewe Pharma: fuorouracil and methotrexate solution for injection (glass particles in vials) in November 2010
Amgen: Procrit and Epogen injections
(glass flakes in vials) in September 2010
Leo Pharma: sodium fusidate (glass particles in vials) in July 2010
Baxter US: Hylenex recombinant (glass flakes in vials) in May 2010
Schering-Plough: Temodar injection (defects in glass vials may compromise integrity of vials) in March 2010.
Find additional recall information on this FDA website: www.fda.gov/Safety/Recalls/default.htm)
Working together, drug and packaging manufacturers can examine potential interactions among a drug product’s formulation characteristics —e.g., pH, buffer type, and ionic strength — in relation to the area of contact with its container and environment. When a risk of glass attack or breakage is identified, an alternative high-performance polymer material (such as a cyclic olefin) might offer a beneficial solution.
Glass in Container–Closures
Future trends toward individual therapies will drive manufacturing and distribution techniques in a new direction. It has been predicted that novel products such as imaging for diagnostics, new antibody treatments, biomarkers, tissue engineering, nanocarriers, and gene-based and stem-cell therapies will reach the market by 2020 (6). Glass may not be the logical or preferred choice for some innovative and high-value products. Technological advancements in therapeutics and diagnostics should be matched by innovations in the containment and delivery systems.
During drug-product development and manufacturing, all materials that will be in contact with the product should be regulatory-compliant and evaluated for protection, function, compatibility, and safety. Initial assessment of these materials should include an understanding of the risk they pose to quality.
Glass has a long history of use as a container. Over time, it has evolved to serve new functions across a broad range of fields and activities. Changes in the glass industry have been driven by greater standardization, complex global supply chains, and ultimately the demand for durability with modern pharmaceuticals and biomolecules. The constituents of glass and conditions for shaping container systems can affect its overall quality and suitability for use with a given biopharmaceutical product. Glass is not chemically inert, and its physical properties are not suitable for all applications necessary to meet pharmaceutical requirements of the 21st century.
One risk factor associated with glass is the variability inherent in its raw materials. Their sources can give rise to differences in a glass container’s chemical make-up and quality. The basis of glass is silica (SiO2) from naturally occurring sand or rock that can originate from around the world. To process glass, fluxes such as sodium or potassium can be added to lower the silica melt temperature, and stabilizers can be used to improve durability and reduce potential for glass to breakdown into its constituent parts. Although the major proportion of glass is SiO2, a number of naturally occurring minor trace elements can affect the glass forming process and have an immeasurable impact on the quality of drug products in the resulting containers. Variation in glass-batch constituents and forming conditions can affect the quality, durability, workability characteristics, and aesthetics with an overall impact on short-term and/or long-term drug product quality (7).
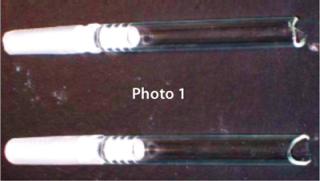
Risks Lead to Costly Recalls: By law, containers must not react with or add to the drug products inside them in any way that alters safety or quality. Increasingly, the risk of glass delamination is becoming a factor to be considered when developing a drug product (8). Concerns related to glass include breakage during filling or transport (Photo 1), potential leachables, pH shifts from glass dissolution, p
otency loss when a drug adsorbs to its container, formation of interaction products or precipitants, and occurrence of pitted surfaces (Photo 2) and potential for glass delamination. Interactions between drug products and glass or the silicone oil used to lubricate glass-based syringes can cause protein aggregation (Photo 3).
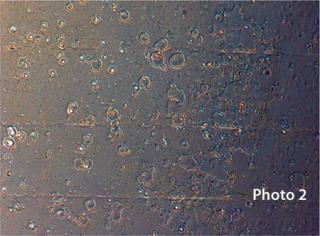

Glass flakes — also known as siliceous flakes or lamellae — are the result of glass delamination that can occur slowly over time in the presence of neutral, slightly acidic, or basic solutions. When sodium and potassium ions are solvated from a glass surface, the SiO2-enriched layer can delaminate to form glass flakes. You can see the onset of glass delamination through microscopic examination of the interior vial/syringe surface for signs of pitting. But the effect is intermittent and difficult to predict with certainty.
For a pharmaceutical manufacturer facing a potential recall, the total cost of quality can dramatically increase the price of manufacturing a drug product. Performing risk assessments based on factors such as the physical, functional, and chemical characteristics of a container–closure system — as well as interactions with the drug product — enables selection of the most suitable option. And sometimes, glass may be too risky for use with particular products, as demonstrated by the recent number of glass recalls.
Polymer Material Options
Polymer materials have been and can be used safely as packaging components for parenteral drug products, providing that critical criteria are met. Relevant properties for parenteral primary container–closure systems include optical clarity, high strength, heat resistance, low extractability, long-term stability, dimensional consistency, and minimal particulates. These systems must present a barrier to water and/or oxygen and a lack of cosmetic defects.
The polyolefin family of polymers — specifically polypropylene and cyclic olefin polymers — is used frequently for parenteral applications, but the type and grade of polymer must be considered carefully to ensure drug product quality and mitigate the risk of unexpected interactions. These materials can pose similar risks to drug product quality, safety, and efficacy as those of glass, but the behavior of polymers is quite distinct. A major issue for both types of material is the potential for drug product to leach chemical constituents from the components used in their manufacture, storage, or delivery.
Leachables: Organic and inorganic species can leach out based on direct or indirect contact of materials during manufacture, storage, or shipping. Leachables can threaten patient safety even at low to trace levels because they can be toxic, form reaction products, or lower product efficacy. Risk-assessment for leachables should begin during drug-product development and continue throughout stability studies to determine the presence or absence of troubling compounds. The degree of evaluation should be commensurate with the stage of drug development. Tactics for qualifying leachables involve gaining comprehensive knowledge on the chemistry and extractability of critical component materials.
Physical and functional properties are intrinsic to a polymer’s molecular structure and chemical make-up. The particular desired combination of properties can be enhanced with additives, processing aids, and/or copolymers. The polymerization process and composition of ingredients can add to chemical complexity and the capacity for chemical constituents to diffuse out of their polymer matrix and dissolve or partition into a drug product. The mobility of constituents in a polymer matrix (potential leachables) relative to different solvent systems can be explored early in drug development. Aqueous-based products are very compatible with polyolefins, and the chromatograms in Figure 1 illustrate some chemical constituents that can be extracted with polar solvents under exaggerated conditions. With those solvents, the extractability of polypropylene is higher for polyolefins than for Daikyo Crystal Zenith (CZ) polymer, a high-performance cyclic olefin polymer developed by Daikyo Seiko, Ltd. (www.daikyoseiko.jp) solely for the purpose of drug containment and delivery.

Figure 1: ()
Choosing the proper materials to carry out further studies at an appropriate stage should be timely and cost efficient. A comprehensive set of extraction tests using multiple solvents and analytical techniques eventually are needed to develop a strategy for leachables qualification. Monitoring for leachables throughout drug product stability and clinical trials can ensure the absence or limit the amount and impact of leachables, degradation by-products, or reaction products associated with chemical entities originating from polymer components. A packaging system found acceptable for one drug product is not automatically assumed to be appropriate for another (9). Selection of appropriate materials early in drug development will narrow a company’s risk of discovering unanticipated package–product i
nteractions that could lead to poor drug quality and drug product rejects at later stages.
Cyclic Olefin Polymers: A cyclic olefin polymer (COP) such as the CZ brand is a high-performance material possessing valuable properties necessary to manufacture, store, protect, and deliver drug products without many of the weaknesses inherent to glass. It is especially suitable for biologic applications and was developed specifically for the challenging needs of this market.
Cyclic olefins are modified from general olefins with enhanced performance characteristics through use of cyclic olefin monomers and unique polymerization methods. The synthesis allows for better control of their molecular structure, with fewer side products and less need for performance additives. Coupled with a precision molding process that is optimized for the resin’s characteristics and end-user needs, the capabilities of this material offer advantages to the pharmaceutical and biopharmaceutical industries. This class of polymers has few ingredients and offers the desired properties of transparency, break resistance, moisture barrier, and sterilizability. High glass-transition temperatures and low shrinkage make them suitable for manufacturing materials to be used in parenteral container–closure systems.
The optical transmission for COPs is much higher (>90%) than for other plastics (10). The mechanical and thermal properties are well suited for subzero application so that COPs exhibit high strength and impact resistance. According to Xeonex Corporation (www.xeonex.com), their flexural strength is three to four times greater than that of polypropylene, and their tensile strength can be up to five times greater depending on grade. Another important requirement for pharmaceutical applications is dimensional stability, and COP resins are shown to have high dimensional tolerances due to their thermomechanical properties (10). The COP material is physically and chemically stable, with relatively few constituents presenting low potential for drug product interactions.
The manufacturing process for CZ systems in particular is optimized to include cleanroom manufacturing, 100% vision inspection, and presentation in a sterile format compatible with established drug-filling equipment.
Protecting Biologics
When selecting materials to be used in contact with biologics, significant issues must be considered. Strength without brittleness is critical to mitigate breakage risk. Potential for gas permeation and absorption or interaction with drug products needs to be qualified for intended use. Protein aggregation is a major issue that can be mitigated by choosing appropriate contact materials. High-performance COPs have been shown to mitigate risk of protein aggregation during storage and shipping. Figure 2 illustrates the effect of end-over-end rotation at room temperature on aggregation of a therapeutic monoclonal antibody in 1-mL long glass and CZ syringes (11). Solution turbidity measured by absorbance at 350 nm indicated where aggregation occurred in glass prefilled syringes.
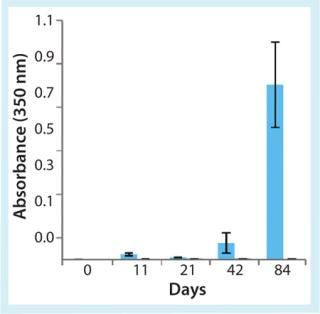
Figure 2: ()
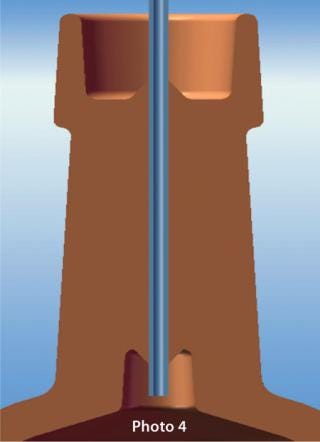
Proteins are also sensitive to inorganic elements, especially tungsten oxide (a residual that comes from the channel for inserting the needle in a prefilled syringe). Needles can be inserted during the forming of CZ syringes without the use of tungsten pins, which eliminates tungsten residue from the system. Photo 4 illustrates a prefillable syringe fabrication with needle inserted.
Another characteristic of COPs is their moldability, which allows for customized shape and size. Once a COP is qualified as suitable to be in contact with a drug product, that material can be used in a number of formats that meet the changing requirements of the product’s life cycle. This enables development of a fully integrated containment and delivery system free from the dimensional and functional limitations of glass. Vial-based container–closure systems can be used to store bulk drug substances in a range of volumes for preclinical trials. Prefillable syringes formed from the same materials then can take the product to market, which reduces the need for materials testing and qualification for multiple container–closure configurations. That can lead to efficiencies and greater understanding of a product throughout its life cycle.
The biopharmaceutical industry is looking for more innovative solutions for container–closure and delivery systems for regulatory compliance as well as to meet the future packaging needs of its products. Realizing critical requirements for drug-product contact materials, manufacturing processes can be optimized using high-performance polymers for safe and compatible container–closure systems.
About the Author
Author Details
Corresponding author Graham Reynolds is vice president of marketing and innovation, and Diane Paskiet is associate director of scientific affairs at West Pharmaceutical Services, Inc., 101 Gordon Drive, Lionville, PA 19341; 1-610-594-2935; [email protected]; www.westpharma.com.
REFERENCES
1.) 2011. Breakage and Particle Problems in Glass Vials and Syringes Spurring Industry Interest in Plastics. IPQ in the News.
2.) 2011. American Regent Initiates Voluntary Nationwide Recall of Calcium Gluconate Injection, USP, 10%, 100 mL Pharmacy Bulk Package Due to Particulates, US Food and Drug Administration, Rockville.
3.) 2011. American Regent Initiates Voluntary Nationwide Recall of Concentrated Sodium Chloride Injection, USP, 23.4%, 30 mL Single Dose Vial Due to Particulates, US Food and Drug Administration, Rockville.
4.) 2011. American Regent Initiates Nationwide Voluntary Recall of Methyldopate HCL Injection, USP 5 mL Single Dose Vial Due to Glass Particulates, US Food and Drug Administration, Rockville.
5.) 2011. Advisory to Drug Manufacturers: Formation of Glass Lamellae in Certain Injectable Drugs, US Food and Drug Administr
ation, Rockville.
6.) 2011. Pharma 2020: Supplying the Future, PricewaterhouseCoopers, New York.
7.) Cummings, K 2002.A History of Glass Forming, University of Pennsylvania Press, Philadelphia.
8.) 2011.Control of Components and Drug Product Containers and Closures US Code Fed. Reg, US Food and Drug Administration, Rockville.
9.) CDER/CBER 1999. Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics — Chemistry, Manufacturing, and Controls Documentation, US Food and Drug Administration, Rockville.
10.) Niles, WD, and PJ. Coassins. 2008. Cyclic Olefin Polymers: Innovative Materials for High-Density Well Plates. Assay Drug Develop. Technol. 6:577-590.
11.) Waxman, L, and V Vilivalam. Evaluation of End- Over-End Rotation/Agitation of Protein Solutions in Prefilled Syringes Made from Glass or Plastic as a Preliminary Indicator of Protein Aggregation:19-21.
You May Also Like






