A Practical Method for Resolving the Nucleation Problem in LyophilizationA Practical Method for Resolving the Nucleation Problem in Lyophilization
Given the prevalence of lyophilization and the growing pipeline of sensitive biological drugs requiring stabilization, pharmaceutical development and manufacturing personnel need complete, reproducible control over the operation, scale-up, and transfer of their lyophilization processes. To address the nucleation problem, Praxair has developed a step-change technology that adds consistent control to the freezing step of lyophilization. This low-capital, plug-and-play option can be readily implemented on most existing freeze-dryers with minor equipment additions and controls integration. Adoption of the technology requires no changes to existing drug formulations and has minimal impact on established lyophilization protocols. This truly enables the broad application of quality by design (QbD) to lyophilization and provides pharmaceutical manufacturers with a novel means of addressing capacity, yield, and quality issues for this critical fill-and-finish operation.

Figure 1:
PRODUCT FOCUS: ALL FREEZE-DRIED PRODUCTS
PROCESS FOCUS: Downstream process development, formulation, fill–finish
WHO SHOULD READ: PRODUCT AND PROCESS DEVELOPERS, FORMULATORS
KEYWORDS: FREEZE DRYING, QBD, PROCESS CONTROL, MANNITOL
LEVEL: BASIC
Background
Pharmaceutical and biopharmaceutical manufacturers often must dry their products to meet shelf life targets by inhibiting chemical, microbiological, and physical degradation pathways that occur in the presence of moisture. A wide variety of drying methods have been used including spray drying, freeze-drying (lyophilization), vacuum drying, and fluidized-bed drying. Lyophilization has become the preferred method for sensitive, high-value pharmaceuticals because it enables moisture removal at relatively low temperatures under easily maintained sterile conditions. Furthermore, the technique often imparts the valuable product attribute of rapid reconstitution at the point of use. Lyophilization has a long history of regulatory acceptance and will continue to occupy a prominent role in pharmaceutical fill–finish operations for decades to come.
In a typical freeze-drying process, multiple vials containing a liquid drug formulation are loaded on temperature-controlled shelves within a sterile chamber and cooled to low temperatures until completely solidified. Following that freezing step, chamber pressure is reduced and shelf temperature adjusted to enable removal of the frozen solvent (drying) through sublimation in a step termed primary drying. When sublimation is complete, the shelf temperature is raised for secondary drying to remove additional un-frozen solvent still bound to the solid product. When sufficient solvent has been removed, the drying process is concluded by stoppering the vials or bottles in the chamber, generally under a subambient pressure of inert gas. The final dry product is called a cake and usually occupies roughly the same volume as the initial liquid fill because of its high porosity. This whole process usually takes a number of days to complete.
Nucleation: Many technical issues surrounding the freeze-drying process have been addressed over the past several decades. Better understanding of critical formulation characteristics and cycle conditions combined with consistent equipment advances has delivered incremental improvements in development and product quality. However, not all steps of a commercial lyophilization process are adequately controlled today. In particular, nucleation of the freezing step — at the very beginning of a lyophilization cycle, thus a key determinant for the rest of the process — currently occurs stochastically (randomly).
To simplify discussion, our description of the nucleation phenomenon focuses on aqueous solutions, which are most commonly found in the biopharmaceutical industry. During freezing, the aqueous solution in each vial is cooled below its thermodynamic freezing point and remains in a “subcooled,” metastable liquid state until ice nucleation occurs. Nucleation denotes the onset of crystallization, when water molecules dispersed in solution first gather to create stable clusters (nuclei) on the nanometer scale. Addition of subsequent water molecules to those nuclei constitutes crystal growth, and nucleation and growth processes continue under cooling until a formulation is completely solidified.
The probability that such a solution will spontaneously nucleate and begin freezing increases with deeper subcooling and the presence of contaminants, additives, structures, or disturbances that provide a site or surface to catalyze formulation of nuclei. In clean CGMP manufacturing environments, formulations are relatively free of nucleation-inducing contaminants. So the range of nucleation temperatures across a set of vials will distribute randomly between the formulation’s thermodynamic freezing point (near 0 °C) and a temperature as low as −30 °C. Figure 1 shows an example of this stochastic nucleation behavior. Note the widely differing product temperature histories.
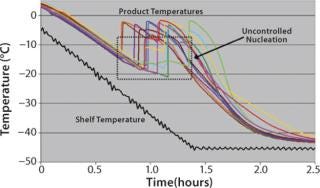
Figure 1: ()
Adverse Effects of Uncontrolled Nucleation
Manufacturing Cost and Capacity: Lyophilization cost and capacity are severely limited by an inability to effectively control nucleation on a commercial scale. The significant subcooling that occurs in most vials before nucleation leads to formation of smaller ice crystals. It is generally recognized that smaller ice crystals reduce the primary drying rate because mass transfer is limited through the small pores they leave behind as they sublimate. It is estimated that for every degree increase in nucleation temperature, there is a 1–3% decrease in drying time (1,2). As a result of subcooling, the primary drying step must be run excessively long to accommodate the slowest-drying vials (those vials that nucleated at the coldest temperatures). Without uniform nucleation at warmer temperatures, lyophilization cycles are significantly longer than would be ideal — increasing manufacturing costs (energy, maintenance, labor) and reducing capacity. Longer cycles also mean increased risk of product loss because high-value product may remain in a freeze-dryer for extra hours or days.
Product Yield: Although lyophilization is considered a relatively gentle preservation method, the inherent freezing stresses can negatively affect product yield, particularly for sensitive biologics. Ice formation may damage an active pharmaceutical ingredient (API) directly through physical or interfacial interactions or indirectly through severe changes in the osmotic forces or solute concentrations it experiences. Because nucleation controls the kinetics and structure of ice formation, it can significantly influence those stresses. For example, deeper subcooling before nucleation causes relatively smaller ice crystals, which possess greater surface area on which proteins may denature and aggregate. The challenge of obtaining high protein yields is thus exacerbated by a lack of nucleation control.
Ice nucleation also influences other crystallization processes during the freezing step that can directly affect product yield. For example, it is well known that formulations containing certain common crystallizing excipients (e.g., mannitol) can undergo phase transitions during freezing or primary drying — enough to induce physical stresses sufficient to crack containers. Though they are poorly understood, the phase transitions appear to involve an excipient crystallizing from an amorphous form or recrystallizing from a less stable polymorph. When nucleation behavior is highly random, the likelihood of producing such undesirable excipient phases during freezing is substantially increased.
Product Quality: Uncontrolled nucleation introduces significant heterogeneity because nucleation behavior strongly influences product microstructure, rate of drying, crystallization of excipients, and the local preservation environment of an active ingredient. Such processing heterogeneities can translate into vial-to-vial differences in important final product attributes such as API activity, moisture content, cake appearance, and reconstitution time.
Working in a QbD Framework: Development scientists face many challenges in creating adequate formulations and lyophilization cycles for new products. It can often take many months to develop a successful method with a reasonable cycle time that preserves sufficient API activity and provides other desired product properties within specified uniformity. The increasingly emphasized QbD regulatory framework encourages rigorous understanding of the effects of all critical process parameters on product attributes and requires the use of process controls to ensure that those parameters remain within acceptable ranges. An inability to control nucleation expands the range of critical process parameters and fundamentally undermines the deployment of science-based QbD principles in lyophilization. Thus extra formulation efforts and nonoptimal lyophilization cycles are necessary to accommodate differences in nucleation behavior between vials in a given manufacturing environment — as well as the differences among various laboratory and commercial environments when lyophilization processes are scaled up or transferred.
Previous Efforts to Control Nucleation
Stochastic nucleation is a well-recognized gap in lyophilization process control. Significant work has been gone toward elucidating the problem and exploring various control methods and their potential for commercial application. However, past efforts have yielded no process that has been implemented on a commercial scale.
Additives: In general, all additives/contaminants have the potential to serve as nucleating agents. The most commonly investigated agents include silver iodide, Pseudomonas syringae bacteria, and adventitious environmental particulates. The use of additives is not typically acceptable or desirable for lyophilization of FDA-regulated and approved pharmaceutical products. Additives provide insufficient control over time and temperature when individual product vials nucleate and freeze; they serve only to increase their average nucleation temperature.
Ice Fog: Ice crystals can themselves act as nucleating agents. In the “ice fog” method originally described by Rowe in 1990 (3) and demonstrated at laboratory scale by Rambhatla et al. (2), a humid freeze-dryer is filled with a cold gas to produce a vapor suspension of small ice particles. Those ice particles induce nucleation when they contact the fluid interface inside each vial. This method faces challenges in controlling the nucleation of all vials simultaneously at a desired time and temperature for commercial scale freeze-dryers. Uniform distribution of the ice suspension may be difficult, and ice particle transport rates may be different for vials in different locations. Successful commercial-scale implementation of the ice-fog method has yet to be reported.
Vial pretreatment by scoring, scratching, or roughening has also been used to lower the degree of subcooling required for nucleation. This works by producing surface defects or glass nanoparticles that can catalyze nucleus formation. As with other additives, glass nanoparticles are generally undesirable for pharmaceutical products. Vial pretreatment imparts no control over the time and temperature when individual vials nucleate and freeze, but instead only increases their average nucleation temperature overall.
Ultrasound: The well-established ability of ultrasonic vibration to induce nucleation in subcooled solutions has been applied to lyophilization applications at small scales (4). It is generally believed that disturbances caused by the rapid growth and collapse of gas bubbles under transient cavitation trigger nucleation. The major challenges of implementing this method in a commercial-scale freeze-dryer — with sufficient uniformity and without compromising equipment cleanability — have not yet been met.
An electrofreezing method has also been used to induce nucleation in subcooled solutions (5). This is generally accomplished by delivering relatively high electric fields (~0.01 V/nm) either continuously or pulsed between narrowly spaced electrodes immersed in the solution to be freeze-dried. The need for individual electrodes in each vial make this method truly impractical for use in commercial pharmaceutical manufacturing. Also, electrofreezing cannot be applied directly to formulations that contain ionic molecules (e.g., NaCl).
A Practical Method for Solving the Problem
Praxair has developed a novel and scalable method for controlling nucleation of the freezing step in commercial lyophilization. This step-change process manipulates pressure to uniformly and simultaneously induce nucleation in all vials within a freeze-dryer. The vials and their contents are not contacted or touched by anything except inert gas, which is already present in the process and gets evacuated from the vials during the lyophilization cycle.
Through the use of such pressure manipulation, uniform nucleation control has been demonstrated both in laboratory (1-m2 shelf area) and small commercial-scale (5-m2 shelf area) freeze-dryers for a wide range of formulations. Repeated tests with containers ranging from 2-mL vials to 60-mL bottles to bulk trays have demonstrated that nucleation of aqueous solutions can be precisely controlled to within 1 °C of their thermodynamic freezing points. Figure 2 shows an example of the improvement possible with this method. Note that by contrast with Figure 1, the product temperature histories created by controlling the nucleation for each vial are nearly identical.
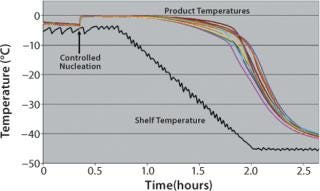
Figure 2: ()
Cake Microstructure: The microstructure of cakes lyophilized with conventional stochastic nucleation and Praxair’s nucleation control method have been examined with scanning electron microscopy (SEM), polarized light microscopy, and gas adsorption. All three methods verify the expectation that controlled nucleation at warmer temperatures produces substantially larger pores than stochastic nucleation does. Figure 3 compares pore dimensions for lyophilized 5 wt% sucrose, for example.

Figure 3: ()
Drying Rate and Uniformity: Various potential benefits of controlling nucleation have been demonstrated through comparative studies of model formulations lyophilized with and without Praxair’s nucleation control method. Drying times can be dramatically reduced by producing larger pore sizes through controlled nucleation. The primary drying of 5 wt% sucrose was completed in just 86 hours using controlled nucleation compared with 118 hours for stochastic nucleation. Similarly, in a study of 3 wt% mannitol with primary drying interrupted for both stochastic and controlled nucleation after 11 hours, it was observed that 19% more moisture was removed using controlled nucleation. Controlled nucleation also reduced the absolute standard deviation in percent residual moisture for the mannitol cakes from 4.6% to 2.1%, demonstrating improved product uniformity.
Vial Cracking: As mentioned above, mannitol is commonly recognized for breaking vials through crystallization irregularities that occur in the freezing step. Such problems are generally exacerbated by high-mannitol concentrations, high fill volumes, and fast cooling rates. Wherever vial cracking was observed after freezing mannitol-containing formulations with conventional stochastic nucleation, the use of controlled nucleation completely eliminated the cracking problem.
Protein Aggregation and Activity: Repeated tests have demonstrated that Praxair’s controlled nucleation technology has been effective at reducing protein aggregation and improving product activity. These effects have been explored using the protein lactate dehydrogenase (LDH) as a model with dynamic light scattering (DLS), size-exclusion chromatography (SEC), and enzyme activity assays.
LDH sourced from two different vendors was combined at a concentration of 1, 0.25, or 0.05 mg/mL with either 12.5-or 100-mM citrate (pH 7.5) or Tris (pH 7.5) buffer to make 24 different test formulations. They were subjected to a single freeze–thaw cycle in a lyophilizer with ramp rates of ~1°C/min. DLS and SEC confirmed that LDH experienced severe aggregation in 16 of 24 cases (67%) when nucleation was stochastic but only six of 24 cases (25%) when it was controlled. Activity assays for 1-mg/mL LDH in 5 wt% mannitol after freeze–thaw in a lyophilizer indicated a 39% loss of activity for stochastic nucleation but only a 22% loss for controlled nucleation. So it is possible to substantially mitigate freezing stresses on proteins using controlled nucleation to optimize the kinetics and structure of ice crystallization.
REFERENCES
1.) Searles, JA, JF Carpenter, and TW. Randolph. 2001. The Ice Nucleation Temperature Determines the Primary Drying Rate of Lyophilization for Samples Frozen on a Temperature-Controlled Shelf. J. Pharm Sci. 90:860-871.
2.) Rambhatla, S. 2004. Heat and Mass Transfer Scale-Up Issues During Freeze Drying, II: Control and Characterization of the Degree of Super-Cooling. AAPS PharmSciTech 5:1-9.
3.) Rowe, TD. 1990.. A Technique for the Nucleation of Ice.
4.) Nakagawa, K. 2006. Influence of Controlled Nucleation By Ultrasounds on Ice Morphology of Frozen Formulations for Pharmaceutical Proteins Freeze-Drying. Chem. Eng Process 45:783-791.
5.) Petersen, A. 2006. A New Approach for Freezing Aqueous Solutions Under Active Control of the Nucleation Temperature. Cryobiol 53:248-257.
You May Also Like






