Manufacturing Process AutomationManufacturing Process Automation
January 1, 2012
When you think of futuristic manufacturing facilities and processes, robots might come to mind in a science fiction setting where humans are absent and production lines are directed by computerized drones that perform tasks we can’t even imagine today. As the pace of technology shuttles us swiftly into the real future, however, life-science manufacturers need to pause to examine the purpose of automation and how we can best steer its course.
In bioprocess manufacturing and other industries, technology is growing more powerful and sophisticated for solving problems. General users and implementers of new technologies, however, don’t fully tap into available technology capabilities that exist. Sometimes the complexity overwhelms manufacturing teams and (because of layoffs and consolidation) information technology (IT) departments’ available time. So the options are outpacing the industry’s ability to understand and correctly apply technological solutions to its manufacturing challenges.
Despite advancing technology, the same manufacturing quality problems persist and are being carried into the future while untapped information capabilities may sit idle on your server. To escape this fate, manufacturers need to ensure that future developments are implemented to solve the most critical problems rather than pursued simply for innovation’s sake.
Both types of development are exemplified in everyday life by new smart-phone applications and consumer-based technologies. Ten years ago, people couldn’t imagine the plethora of programs and services such as Yelp, Skype, or the innumerable “apps” for the Apple iPhone. Imagination is the only true limit of future technology evolution, so it is presumptuous to predict what will exist in another 10 years — much less how we will keep pace with future inventions.

Steer the Course of Invention
Technology for process manufacturing grows exponentially. What prevents manufacturers from leveraging available technology for the right problems is threefold and boils down to communication, regulation, and education.
Communication: First, manufacturing industries are home to two kinds of people: manufacturing technology innovators/implementers and users. If users are not fully involved in prioritizing their needs, then innovators may continue down a path of developing and implementing technology that users do not necessarily want or have the time to understand and operationalize. Here are two components at work: need and understanding. Programmers should develop innovations that meet actual needs — rather than simply, “cool tech” looking for a problem to solve.
As Figure 1 shows, the gap widens between the rapid pace of technology and users’ ability to learn new applications in ways that make that technology useful. That gap can shrink when IT professionals and users collaborate to ensure that technology solutions address real-world manufacturing problems — and that adequate training is offered.
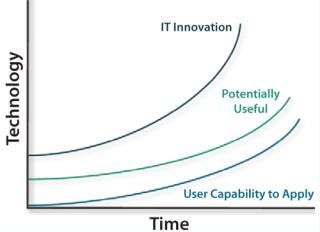
Figure 1: ()
Regulation: Second, life-science manufacturers operate under many regulatory requirements that ultimately drive the agenda for technology development. Over the past decade, quality by design (QbD) has been touted as a means to reach biomanufacturing’s “desired state” of predictability and control. The FDA’s Janet Woodcock has described this desired state: “Manufacturers have extensive knowledge about critical product and process parameters and quality attributes. … Manufacturers strive for continuous improvement” 1,. Some companies have put process analytical technology (PAT) solutions into place, but they may not fully understand them or use them to their full potential.
Education: Finally, the United States is struggling with an education system that may not be adequately preparing the next generation of scientists and manufacturing operators to understand and solve problems with advancing technology. Specialized training is required, as well as a shift to a problem-solving and collaborative type of organization.
In a 2010 article, Nyenrode Business University professor John Psarouthakis shared his perspective on how and why the United States has lost its manufacturing base to foreign nations. One thought-provoking point he made is that today’s first-graders will be entering the workforce in 2020 underprepared as a generation, “which is now struggling with the basic skills of early grammar school” 2,. The article focuses on competitive threats to discreet manufacturing industries, but Psarouthakis wisely proposes, “Very simply, we need to solve three dilemmas: how to develop and deploy new technologies, how to more effectively manage our manufacturing organizations, and how to develop human resources for the future.” Those potential roadblocks to progress intersect for most biomanufacturing organizations and thus need to be considered by companies hoping to move rapidly toward the desired state of QbD and take full advantage of IT’s potential.
Freeing Humans for Critical Tasks
Future manufacturing processes are likely to include increasing levels of automation. If designed appropriately through collaboration, process manufacturing technologies will enable greater process understanding that leads to QbD and automated continuous quality verification (CQV). If employees are freed from repetitive manual tasks, they can focus more on problem solving. The following elements are required to keep pace with technology and leverage it as an agent for progress: cultural change, better process understanding, reallocating human resources, and controlling costs to match benefits.
Cultural Change: Improved training and communication can help technologists and users speak the same language. IT needs to do a better job of training users on what technology is available and how it can be applied to facilitate process improvements. Conversely, users need to tell IT what will be useful and applicable and what is needed to improve their job and business performance. Instead of operating cut off from one another in “silos,” technologists and business users can instead work toward common goals on a common platform that involves collectively mapping out “where do we go from here?” to help close the gap between them. Organizations can look for learning opportunities and ways to integrate those into curriculums or enlist distance-learning tools that can rapidly reach far-fl
ung users.
Better Process Understanding: Manufacturing problems primarily stem from two sources: making mistakes due to “the human element” and unexpected outcomes due to a lack of process understanding. When the goal of process automation is to reduce the risk of human error, bioprocesses need to be so predictable that they can be automated to work the same way every time. Manufacturing teams can then understand what might happen and how to react to problems as they arise. That relates to QbD’s goal of understanding the boundaries of a process and potential reactions for every action. With improved process understanding, teams can end up moving toward the ideal QbD environment. Such process understanding requires collaboration across geographical and functional boundaries. When appropriately applied, enabling technologies can assist with such work and in the future could lessen the effects of specific manufacturing geographic locations around the world.
Reallocate Human Resources: If manufacturers understand their processes well enough and have IT tools in place to facilitate quick reactions to problems, then some of those processes can be automated to run with fewer people. Biomanufacturing supply chains will be more predictable, with reduced mistakes, and human resources can thus be freed up for other tasks.
Daniel Pink’s bestselling book A Whole New Mind: Why Right-Brainers Will Rule the Future discusses computer automation trends that will replace human jobs with robots and technology. Pink proposes that although computers can replace (and outperform) humans in many tasks, their missing element of empathy can be delivered by humans alone. The empathetic element is what enables humans to retain their roles when creative solutions are required 3,.
In process manufacturing, there will always be a role for the “empathetic” human. With more automation, companies can refocus people on tasks that are more important and productive. But the potential speed of future automation depends on the complexity of what you are trying to automate. The automotive industry has done it well. Robots do much of what people used to do in vehicle assembly, freeing up employees for more value-added, intellectually stimulating, and revenue-generating work. Similar results can be obtained in more complex biomanufacturing, for which automating mundane processes could allow for more focus on drug and process development, for example, in which decisions must be made creatively — by “empathetic” humans.
Controlling Costs to Match Benefits: Return on investment will always be a major consideration in life-science manufacturing. Benefits of automation have to justify associated costs. For technology investments to make smart business sense, a company needs adequate resources and a well thought-out plan for retooling its organization. Implemented technology cannot be overburdensome to its users. Implementation teams need to access the retraining needed to effectively use new technology and determine whether that will lead to a more cumbersome workflow rather than an improved situation. Such teams should understand the total cost of ownership for technology and automation. The costs of retooling organizations and redeploying staff members should not consume all resources freed up by the new system.
When cost–benefit analysis points to automation technologies that are developed and implemented with collaboration among IT teams and users, technology can fall more in line with QbD goals of future process manufacturing facilities. Manufacturing processes must be understood and technologies implemented according to such understanding so that solutions are developed for productive reasons. Only then can organizations truly reduce costs and save time. Cultural shifts are required to keep pace with technology development as companies focus on reallocating human resources to keep their processes on a path of continuous improvement.
Change will enable organizations to correctly apply technological solutions to solve the most important process manufacturing challenges. Supporting a productive QbD culture requires implementing available technology to gain access to data and provide efficient analysis capabilities to allow better process understanding; using increased process understanding to apply the right technologies for monitoring and control; and training people to take full advantage of technologies in place. Ultimately, these combined efforts will free valuable human resources to work diligently on the next phase of life-science innovation.
About the Author
Author Details
Randy Tatlock is manager of customer development at Aegis Analytical Corp., 1380 Forest Park Circle, Suite 200, Lafayette, CO 80026; 1-303-625-2100, fax 1-303-926-1161; [email protected].
REFERENCES
1.) Woodcock, J 2005.Pharmaceutical Quality in the 21st Century: An Integrated Systems Approach, AAPS Workshop.
2.) Psarouthakis, J 2010.The Future of American ManufacturingThe Business Thinker.
3.) Pink, DH. 2006.A Whole New Mind: Why Right-Brainers Will Rule the Future, Penguin Group, New York.
You May Also Like






