Container–Closure IntegrityContainer–Closure Integrity
An increasing number of biopharmaceuticals — including vaccines, stem cells, and proteins — require cold storage to maintain efficacy before use. However, the ability to maintain container–closure integrity (CCI) during cold storage is not completely understood. Concerns about CCI failure have been raised for storage and shipment of such products in rubber-stoppered vials under cold conditions (e.g., −80 °C or on dry ice). Commonly used butyl stoppers are believed to lose their elastic properties below their glass transition temperature (Tg), which poses a risk to sealability (1, 2).

Temporary loss of CCI under cold conditions could allow cold, dense gas from the surrounding low-temperature storage environment to leak into stored vials. Such an ingress of gas could contribute to loss of efficacy of stored drug products due to interaction with the gas as well as vial overpressurization. That occurs when cold, dense gas becomes trapped within a vial as its rubber stopper warms to a >Tg temperature after removal from cold-storage conditions. After the rubber stopper has regained its elastic properties and reseals the vial, headspace pressure in that vial would increase as cold, dense gas inside it warms to room temperature.
Objective
We undertook a cold-storage study to evaluate and compare the CCI of rubber-stoppered glass and Daikyo Crystal Zenith cyclic olefin polymer (plastic) vials stored under cold conditions. Specifically, we evaluated whether selected rubber stopper–vial combinations could maintain CCI at cold-storage conditions below the glass transition temperatures of the selected rubber stoppers.
Methods
We used nondestructive, laser-based, frequency-modulated spectroscopy (FMS) analysis to measure changes in the internal headspace of our vials (Figure 1). We evaluated headspace oxygen concentration using a Lighthouse FMS-760 headspace oxygen analyzer and headspace pressure with a Lighthouse FMS-1400 headspace pressure/moisture analyzer.

Figure 1: ()
Experiment Samples and Conditions
Two Vial Types: 2-mL glass and 2-mL plastic
Three Butyl Rubber Formula Stoppers: D21-7S, 4432, and 4023
Four Storage Conditions:
−20 °C freezer for one week
−80 °C freezer for one week
Dry ice (−78.5 °C) for one week
Dry ice (−78.5 °C) with road and air transport within a 24-hour period
Initial Headspace: 1 atm nitrogen for freezer storage, 1 atm air for dry-ice storage
50 Samples for Each Vial–Stopper Combination: All samples were capped with a Flip-Off seal using moderate compression. And all components were sterilized before assembly.
All vials were confirmed to have CCI at room temperature before placement in their respective cold-storage conditions. We determined a loss of CCI at cold conditions to have occurred if one or both of the following conditions were measured after storage in cold conditions: overpressure of the vial; a change in headspace oxygen levels. For the latter, oxygen would increase in a vial with an initial nitrogen headspace at 1 atm and stored in a low-temperature air environment such as a −80 °C freezer (an ingress of air replaces nitrogen in the leaking vial). Oxygen depletion would occur in a vial with an initial air headspace at 1 atm and stored in a low-temperature carbon-dioxide environment, such as on dry ice (an ingress of carbon dioxide replaces air in the leaking vial).
Results
Figure 2 displays the difference between the headspace oxygen concentration before and after storage under associated cold conditions. For the glass-vial samples, a loss of CCI is defined as an oxygen increase of 1% atm or greater. Figure 3 displays the sample headspace pressures after storage under associated cold conditions. The initial headspace pressure of the vials was 1,013 mbar. We defined an overpressure to be a headspace pressure of 1,150 mbar or greater, which is three standard deviations or more of the analytical measurement higher than the initial headspace pressure.
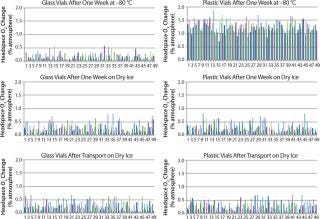
Figure 2: ()
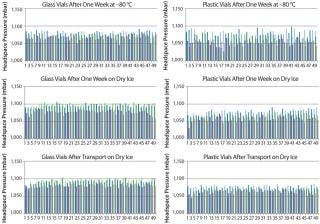
Figure 3: ()
Discussion
As shown above, none of the glass and plastic vial-stopper combinations underwent major changes in headspace oxygen or pressure during cold storage. Therefore, we consider all the vials to have maintained CCI. We do not present the headspace oxygen or pressure results for samples stored in a −20 °C freezer because they were nearly identical to results for those stored in the −80 °C freezer.
In Figure 2, a headspace oxygen increase plotted in the top-right graph from plastic-vial samples stored for a week in the −80 °C freezer is due to permeation effects rather than a leak. We attribute the oxygen increase to samples being stored under normal atmospheric conditions for four days before being placed in cold storage. During that time, air was able to permeate the plastic material walls of the nitrogen-filled samples and increase their oxygen content. We measured representative samples from the sample sets befo
re cold storage, which confirmed that oxygen entered the vials during those four days at room temperature.
We believe that sufficient capping compression — as well as compatible vial and stopper dimensions with regard to the fit between the stopper plug diameter and inner vial neck diameter — counteracts the thermal contraction and glass transition of rubber stoppers at cold conditions. The data demonstrate that conditioned samples evaluated during this study maintained CCI. Additional studies would be needed to verify that similar results could be obtained using different stopper configurations, vials, capping pressures, cold-storage conditions, storage duration, shipping conditions, and vial contents. As demonstrated herein, nondestructive headspace analysis is a straightforward method for generating analytical data to determine maintenance of CCI at low temperatures.
The rubber stoppers we tested are suitable for use with both glass and Crystal Zenith plastic vials for cold-temperature storage (at −80 °C or on dry ice) as well as for transport in dry ice. Both rubber-stoppered glass vials and Crystal Zenith plastic vials demonstrated CCI at temperatures as low as −80 °C, well below the rubber stoppers’ Tg range (between −60 °C and −65 °C).
About the Author
Author Details
Patrick Curran is a process engineer, and Vinod Vilivalam is director of strategic marketing and technical development for the Americas at West Pharmaceutical Services, Inc., 530 Herman O. West Drive, Exton, PA 19341; 1-610-594-2900, fax 1-800-345-9800; www.westpharma.com. Suzanne Kuiper is a European application scientist, and Dr. Derek Duncan is a product line manager at Lighthouse Instruments, LLC, 2020 Avon Court, Suite #2, Charlottesville, VA 22902; 1-434-293-3081, fax 1-434-293-7773; www.lighthouseinstruments.com.Daikyo Crystal Zenith is a registered trademark of Daikyo Seiko, Ltd., from which West has licensed Crystal Zenith Technology. Flip-Off is a registered trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions. Lighthouse FMS-760 and FMS- 1400 are trademarks of Lighthouse Instruments, BV.
REFERENCES
1.) Zuleger, B. 2012. Container/Closure Integrity Testing and the Identification of a Suitable Vial/Stopper Combination for Low- Temperature Storage at –80 °C. PDA J. Pharm. Sci. Technol 66:453-465.
2.) Taylor, J. 2001. Recommendations on the Control and Monitoring of Storage and Transportation Temperatures of Medicinal Products. Pharmaceut. J 267:128-131.
You May Also Like






