September 1, 2011
Picture rows and rows of chicken eggs incubating not to hatch chickens, but to produce vaccines. With the exception of a few products on the market now, most vaccines are still made using this 50-year-old technology. Using chicken eggs to produce vaccines takes about half a year to complete and requires on average one to two eggs to make a single vaccine dose. It is inefficient, labor intensive, time consuming, and subject to contamination. The latter may be the most important problem. In addition to the vaccine you want, contaminants (including other viruses) can get into the product stream.
But there may be a way to modernize our vaccine technology. Rather than producing a virus or a protein to inject into an organism, we can produce a sequence of DNA that has been genetically engineered to express proteins from a specific pathogen when introduced into an organism. The idea is that once the organism’s cells have made the proteins from that DNA sequence, the immune system will recognize them as foreign and launch a response against them.
I’ve been working in DNA vaccines for two decades. At first, as strong believers in a powerful technology, we thought we would have approval in humans in less than five years — but that didn’t happen. Yet this is still a field that I believe will revolutionize the world of vaccines. We still have many challenges to overcome, but it’s clear that we have made significant progress.
Technological Success
Why use DNA vaccines? First, they are fast: decreasing the traditional months for a production run down to weeks. Last year, the industry managed to produce H1N1 flu vaccines in about 30–40 weeks, but that wasn’t good enough. DNA vaccines take four to six weeks to go from gene sequence to vial. The production process is safer, as are the products themselves. We’ve closely examined the potential issue of DNA integration into the chromosomes of recipients. In many safety studies, we’ve never found that to happen. The product doesn’t integrate, and it gets removed from a person’s body over time.
DNA vaccine technology is also less expensive than that for traditional vaccines. It uses bacterial fermentors that can be used for other processes, and DNA vaccines can be made using very simple techniques — even more straightforward than the recombinant DNA technologies used to express protein subunits.
However, all the market successes we’ve had so far have been in animals: horses, salmon, pigs, and dogs (Table 1). The dog melanoma vaccine, in particular, has made a big difference in curing a problem that was previously very difficult to treat. This validates the DNA vaccine technology, and it also shows that we’re almost ready for human vaccines. It demonstrates that this technology is very cost efficient because costs in veterinary applications are necessarily lower than in human medicine.
Table 1: Four veterinary vaccines have been licensed, which validates the technology.

Table 1: Four veterinary vaccines have been licensed, which validates the technology. ()
Historical Overview
In 1990, by pure serendipity Wolf and Felgner discovered in a controlled experiment that injecting DNA in mice produced an immune response (1). That got the field of DNA vaccine research started. A 1993 paper reported on a collaboration between Merck and Vical showing that the technology worked quite efficiently in an influenza model (2). In 1994, David Weiner at University of Pennsylvania filed the first IND for a DNA vaccine. And then my group at Vical developed the first processes to make pharmaceutical-grade DNA (3).

When I entered the field, all the methods used for DNA production were based on the use of ethidium-bromide double-cesium purification, resulting in DNA that could not be injected into humans. So we needed a process that could be scaled up for clinical (and eventually commercial) vaccine manufacturing. Although now it’s pretty obvious to purify kilogram quantities of DNA, fifteen years ago it required a change of mind set for biochemists who were used to purifying protein and getting rid of DNA.
The early 1990s generated a lot of excitement about DNA vaccines, followed by some clinical disappointments — except in animals, where the technology has worked very well. Since then, we’ve seen refinement and optimization of DNA vaccine expression, potency, and immunogenicity. Novel formulations and adjuvants are providing much better protection. There’s been optimization at the genetic level: e.g., codon optimization to increase T-cell response. And heterologous prime-boost vaccination is making significant inroads (4). Harriet Robinson’s group at Emory University in Atlanta, GA, has achieved good results in primates using DNA for priming and recombinant modified vaccinia Ankara (MVA) virus.
Manufacturing Progress
Meanwhile, diligent work in manufacturing technologies has addressed yield and stability issues. These methods are scaling up successfully to at least 1,000 L or more. And a 1,000-L process can yield millions of vaccine doses, which allows for commercial production in a relatively small plant. Disposable bioreactors do not work for DNA vaccine production because of oxygen-transfer limitations. But single-use technologies do play a significant role in downstream processing to reduce cleaning validation work, improve manufacturing efficiencies, and lower overall costs.
Major progress in formulation and lyophilization technologies is making DNA vaccines more shelf stable. The cold chain is a major issue for all vaccines, especially those sent to developing countries. Not everyone has the necessary refrigeration or freezing equipment to store sensitive liquid formulations.
Case Study: Figure 1 shows the kinds of product yields we’ve had over the years at Althea. It begins with something we thought was pretty good at the time! Now we regularly see g/L product titers. Human-vaccine lots we’ve produced range mainly from preclinical to phase 2 scale. We’ve worked with clients from Europe, the United States, and the Asia–Pacific region, to produce preclinical, phase 1, and phase 2 products.
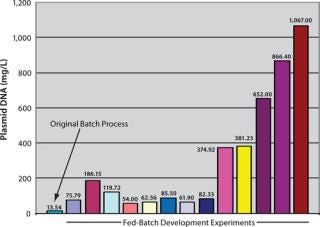
Figure 1: ()
Our process development team has progressively been able to increase manufacturing yields through a range of fermentors from the 30-L scale to the 100-L scale and now at the 1,000-L level. We’re still working in the clinic to make sure that we have the right human-vaccine product before we can go to market. But in terms of manufacturing, we’re ready. That’s not the limiting step.
A Brave New World
Are DNA vaccines ready for prime time (5)? It’s taken nearly 20 years, and development is ongoing. Things didn’t work the way we initially imagined they would, but that’s similar to the story of monoclonal antibodies (MAbs). After 20 years, by the time many investors didn’t believe in MAbs anymore, the technology finally worked — and that changed the pharmaceutical industry. I see this coming for DNA vaccines as well. Major progress has been made not only in basic science and product development, but also in delivery and manufacturing. Collaboration among industry, academic laboratories, and governments has facilitated much of that progress. Clinical trials are ongoing to assess expression, potency, and immunogenicity. Once we get one success, then many others will follow, and it will be a revolution.
This article is adapted from a presentation given at Interphex in New York, NY, March 2011 in a lunchtime session moderated by BPI’s editor in chief, S. Anne Montgomery
About the Author
Author Details
Magda Marquet, PhD, is cofounder and cochair of the board of directors for Althea Technologies, Inc., 11040 Roselle Street, San Diego, CA 92121; 1-858-882-0123, fax 1-858-882-0133; [email protected]
REFERENCES
1.) Wolf, Felgner. 1990. Direct Gene Transfer into Mouse Muscle In Vivo. Science 247.
2.) Ulmer,. 1993. Heterologous Protection Against Influenza By Injection of DNA Encoding a Viral Protein. Science 259.
3.) Horn, Marquet. 1995. Cancer Gene Therapy Using Plasmid DNA: Purification of DNA for Human Clinical Trials. Hum. Gene Ther. 6.
4.) Robinson, HL 2003. Prime Boost Vaccines Power Up in People. Nat. Med. 9:642-643.
5.) Kutzler, MA, and DB Weiner. 2008. DNA Vaccines: Ready for Prime Time?. Nat. Rev. Genetics 9:776-788.
You May Also Like






