Establishing Strategic Supplier Partnerships to Facilitate Manufacturing SuccessEstablishing Strategic Supplier Partnerships to Facilitate Manufacturing Success
In November 2009, Talecris Biotherapeutics announced an ambitious US$269 million expansion of its Clayton, NC, manufacturing facility. The company was subsequently purchased by Grifols, Inc. in 2011. Constructing a new facility with a state-of-the-art manufacturing process intended to generate clinical products involves top-notch project management, exceptional fortitude, and numerous supply chain decisions. Suppliers are often relied on to provide standard product support. When used effectively, they can be an invaluable resource beyond basic services. Additional support may include engineering expertise, custom device development, and even regulatory insight.
Forming strategic partnerships with the right suppliers is important to preventing critical-path project delays. When a biopharmaceutical company builds a new manufacturing facility and implements a novel process design, not all suppliers are willing or able to provide the necessary product support in the time required. As a leading provider of plasma-derived therapeutic components, Grifols relies on quality partnerships to achieve its complex production goals. Below is a case study of the key partnership between Grifols and single-use systems (SUS) supplier Charter Medical, Ltd. (CML), of Winston-Salem, NC. The output of this alliance was development and implementation of a crucial SUS processing component in time to keep the new facility’s opening on schedule.

Background
Headquartered in Barcelona, Spain, Grifols is a multinational healthcare company. This principal producer of blood-plasma–based products also supplies devices, instruments, and reagents for clinical testing laboratories. The company is a major world supplier of intravenous immunoglobulin (IVIG), albumin, blood factor VIII, and other plasma-derived products. The global bioscience division of Grifols is committed to hemotherapy and producing life-saving protein therapies, with a standing mission to improve the health and well-being of patients around the world. These hemotherapeutic products are derived from human plasma, the liquid portion of human blood that contains essential proteins for treating rare, chronic, and life-threatening conditions (immune deficiencies, bleeding and blood-clotting disorders, alpha-1 antitrypsin deficiency, and so on) (1).
Grifols built a leading-edge plasma fractionation manufacturing facility in Clayton, NC, where the company is implementing and upgrading the proprietary centrifugation process it uses to obtain the plasma fractions. During installation qualification (IQ), Grifols encountered an issue with an SUS component necessary for the processing and storage of the precipitated fractions. Process development engineers reached out to CML for assistance.
CML has an ISO 13485 certified manufacturing facility located in Winston-Salem, NC. The company’s vital fluid business serves bioprocessing and medical device customers. It designs and supplies both standard and custom SUS for processing cell-based and biologic fluids in biotech and clinical applications.
Plasma Fractionation
Plasmapheresis is the technique used to separate plasma from other blood components. José Antonio Grifols Jr. developed this technique, a process of extracting human plasma while simultaneously reinjecting red blood cells back into a donor, based on the work of researchers Abel and Co Tui. Following collection, the blood plasma is shipped to a Grifols processing facility for fractionation and subsequent purification.
Grifols’ manufacturing process is based on the Cohn fractionation method developed by E.J Cohn during the World War II mainly for production of albumin (2). This process uses the combined effect of pH, ionic strength, and organic solvent on protein solubility with the addition of centrifugation and bulk freezing steps to obtain a number of fractions (1, 3, 4). The standard human plasma fractionation process yields five distinct major fractions.
Fluor Enterprises Provides EPC Services
In planning to significantly expand manufacturing capacity, Grifols selected Fluor Enterprises, Inc., as a partner in the engineering, procurement, construction management, and commissioning (EPC) of its new fractionation facility (NFF) project in Clayton, NC. Augmenting an existing facility there, the project included a 160,000-ft2 fractionation expansion that provides a 43% increase over the company’s previous plasma processing capacity. The project included new centrifuge technology, expansion of existing plant utilities and systems, and refit of an existing ethanol fractionation facility. Fluor provided an integrated EPC approach using modular construction expertise that facilitated an aggressive schedule date.
The Challenge: Grifols receives blood plasma as a raw material and fractionates it to manufacture therapeutics. The target patient population is typically small, but reliable supply is critical because patients rely heavily on such products. This expansion brings the Clayton facility’s fractionation capacity from 4,000,000 L to 6,000,000 L. As in biotechnology operations, high-grade stainless steel is required for process equipment and clean utilities.
Fluor’s Solution: Grifols began working with Fluor on conceptual design and preliminary engineering in 2009. The site was cleared by spring 2010, so steel erection commenced in early summer. Construction was complete in early 2012, when the facility entered its commissioning and qualification phase. In addition to on-site resources, Fluor involved its life-science experts based in Greenville, SC. This project involved advanced modular design and construction techniques and a concurrent-build model rather than sequential construction (in which a building is first constructed before process units are installed). Both the NFF structure and processing units were built simultaneously to shorten the overall schedule.
For example, precipitation super-skids were designed before the building itself. Too large to be transported by truck, those units were assembled on site. Completed assemblies were then transferred and installed for testing to ready them for commissioning and start-up. This facility is projected to receive licensure from the FDA Center for Biologics Evaluation and Research by the end of 2014, ensuring that patients worldwide have an uninterrupted supply of high-quality blood therapies.
Video:http://www.fluor.com/about_fluor/Pages/videos.aspx?channel=4&videoid=115
Ultimately, a fractionator’s goal is to obtain the highest yield and purity possible for each precipitated product. To achieve that, the Cohn process has been modified several times over the years (1, 3, 5). Variations over the years have included the Gerlough, Hink, Mulford, Kistler and Nitschmann, and Hao methods (5). Some modifications detailed in the Kistler and Nitschmann method led to replacing the centrifugation and bulk freezing steps wit
h filtration and diafiltration (or tangential-flow filtration, TFF). More modern methods have incorporated chromatography (2,6).
The new Grifols facility uses a modified method to the Cohn process as part of its plasma fractionation process. One modification implemented uses a proprietary centrifuge design with a disposable single-use liner to improve production flow and product yield. Given the discharge forces generated by the centrifuge as well as the low temperatures used for freezing and processing/storage, Grifols needed a durable, custom SUS design.
The Process: Centrifugation is used to separate solids from the liquid fraction of human plasma (fractionation). Grifols identified an improved centrifuge that provided several benefits and could be incorporated into the new facility. This centrifuge has a large capacity, clean-in-place and closed processing capabilities, high throughput, and — most important — the ability to capture solids in an integral liner (Figure 1).
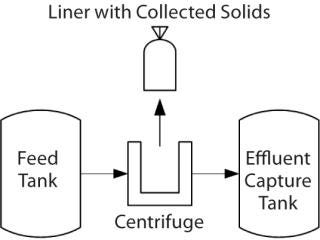
Figure 1: ()
A previous centrifuge design required bowl removal after each run to manually collect solids in the form of a paste. To meet throughput demands, many centrifuges were needed for each batch. The heavy bowls require a lift to remove them from the centrifuge. Paste collected in the bowl is scraped into a bag, which goes to a freezer for storage awaiting further purification. Then the bowl and parts are taken to another room for manual cleaning before they are returned to the centrifuge room for reassembly.
During paste collection, valuable material can be lost as it adheres to centrifuge parts and collection tools. The process also results in a long exposure time before material is transferred to the freezer. With an integral liner, however, the paste is already packaged for freezing when separation is complete and the centrifuge is opened. That reduces loss of the material to paste collection and allows for rapid movement to the freezer, helping to retaining protein integrity. Additionally, with most of the paste removed in the liner, the material in the centrifuge that needs to be removed by cleaning is minimal. Other design features allow the centrifuge to be cleaned in place, which eliminates time-consuming disassembly for a cleaning process.
A liner that withstands hard impacts from ejected paste and cold temperatures (both in the centrifuge and the storage freezer) is essential to the success of this new fractionation facility. The centrifuge operates at many thousands of rotations per minute, creating a strong centripetal force. As a solution passes through, those forces push solids against the bowl wall, where they collect. After a certain volume is collected, that wall opens and solids are dispensed into a larger vessel that contains a liner for paste collection: the paste collection unit (PCU). After several cycles, the larger vessel is opened and the liner removed with the collected paste. Temperature is a factor in protein denaturation, so that paste is frozen as quickly as possible for storage until it is time to transfer it to the purification facility.
Initially the bag used for manual paste collection in the existing process was used for the new collection vessel. It had product-contact–material clearance and can be used at cold temperatures. However, when it was used as a liner for the collection vessel, a problem arose. The film material could not maintain physical integrity during centrifugation. The centrifuge slows down to discharge the solids into the collection vessel, but it is still at a high rotational rate that continues to generate force against the side of the liner. Because of that rotation, this force hits the liner in the stationary collection vessel and twists it against the vessel wall. So the liner would twist and eventually tear along the top, where it was held in place at the joint between the collection vessel and the centrifuge. Thus, Grifols began to search for a material that could withstand both high twisting forces and low temperatures (–70 °C).
CML–Grifols Partnership
Grifols originally evaluated a disposable plastic liner made from 4-mil polyethylene film, obtained from another supplier for the 20-kg PCU application. But initial runs with real product yielded poor results. Due to the centrifuge–tank design, a large lip on the top of the tank makes it difficult to stretch film over without getting wrinkles or folds in the liner along the bottom and sides of tank. Those perturbations trapped air between the liner and the inside of the tank, which resulted in an unsupported condition. At the desired processing temperatures (–5 °C to –20 °C), the liner would tear when product was discharged from the centrifuge because the wrinkles were unsupported against the bottom/sides of the tank. This failure mode was a result of poor fit to the geometry of the vessel as well as limiting physical properties (thickness, tensile strength) of the film.
So an associate within the Grifols technical team who had previously worked with disposable solutions from CML contacted the company in search of a solution. With both standard and custom disposable solutions and experience in freezing and storage of vital fluids, a CML team was invited to Grifols to discuss this demanding application. The team provided several different films and designs for evaluation. And new-product development teams at CML worked with process engineers at Grifols to effectively and quickly perform a series of trial tests of different configurations (in both film and geometry) with the actual tanks.
CML’s disposable Freeze-Pak containers are made from a specialized polyolefin film to be durable and maintain flexibility at cryogenic storage temperatures (–196 °C). The company blended a custom contour tank design with the robustness and durability of its Freeze-Pak film to create a tank liner that effectively fit over the lip of the centrifuge tank while maintaining contact with the tank’s bottom and sides (Figure 2). Parallel to Grifols site visits, CML performed a series of freezing and integrity experiments on this Freeze-Pak tank liner down to –80 °C with 20 kg of frozen water.
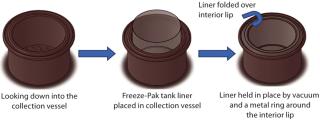
Figure 2: ()
Preliminary results were very encouraging for the fit and integrity of the liner. Therefore, CML and Grifols chose to proceed with the Freeze-Pak film and began necessary steps to qualify the tank liner under current good manufacturing practice (CGMP) requirements. According to internal requirements at both companies, the f
ollowing activities were performed to qualify this new contour tank liner:
Material clearance (Grifols) — material testing (compositional, leachables, and Class VI)
Full design control requirements (CML) — inputs, outputs, design verification/validation, risk analysis, and design transfer to manufacturing
Supplier qualification (Grifols) — audit at CML and quality system review
Process qualification (CML) — installation, operational, and performance qualifications on multiple pieces of equipment, training, inspection, and sampling plans.
CML was first contacted early in November 2011 by Grifols regarding the application described herein. Both companies had agreed on the Freeze-Pak tank liner design by the middle of December. The above CGMP activities were completed by 15 February 2012, and the first 10 commercial liners were delivered on 22 February 2012. This aggressive timeline was required for process validation runs at Grifols to be started in March 2012 (Figure 3). Coupled with significant work required to qualify the new liners, the demanding schedule displays the strong partnership and commitment of both companies in achieving success. The partnership resulted in a custom product with rugged durability and flexibility at the frozen processing and storage temperatures required.
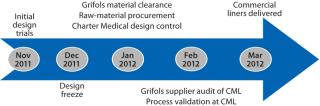
Figure 3: ()
Security of supply for plasma protein therapies is crucial for the health of the population. This is particularly true of the IVIG supply, for which demand is growing. Grifols has made significant investments in increasing its manufacturing capacity to meet the future needs of the patient population. The availability and acceptance of reliable SUS and associated advantages that come with closed systems and processing steps an increase a company’s potential capacity — and give it the ability to react more quickly to market changes. Durability issues of the original single-use design threatened to delay the opening of this new facility. But working closely together with a high sense of urgency, Grifols and CML were able to implement a robust custom solution in time to put the project back on schedule.
About the Author
Author Details
Robert Large is a senior process development engineer at Grifols, Inc., Clayton, NC 27520; Jared Ragone is a new product development engineer, Joe Petrosky is vice president of global sales and marketing, and corresponding author Dominic Clarke, PhD, is the product manager for cryogenic storage and cellular therapy at Charter Medical, Ltd., Winston-Salem, NC 27103. Freeze-Pak is a registered trademark of Charter Medical, Ltd.
REFERENCES
1.) Brodniewicz-Proba, T 1991. Human Plasma Fractionation and the Impact of New Technologies on the Use and Quality of Plasma-Derived Products. Blood Rev. 5:245-257.
2.) Curling, J, and C Bryant. 2005. The Plasma Fractionation Industry: New Opportunities to Move Forward?. BioProcess Int. 3:18-27.
3.) Edsall, JT 1979. The Development of the Physical Chemistry of Proteins. Ann. NY. Acad. Sci. 325:52-74.
4.) Matejtschuk, P, CH Dash, and EW Gascoigne. 2000. Production of Human Albumin Solution: A Continually Developing Colloid. Brit. J. Anesthesia 85:887-895.
5.) Graham, JM, and D Rickwood. 1997.Subcellular Fractionation, a Practical Approach, Oxford University Press, Oxford.
6.) Curling, J 2002. Integrating New Technology into Blood Plasma Fractionation. BioPharm Int:16-26.
You May Also Like






