September 1, 2011
In the early spring of 2009, a new strain of H1N1 influenza emerged and swept across the globe more rapidly than vaccine producers could keep pace. By the time the pandemic abated in February 2010, the US Centers for Disease Control (CDC) estimated that between 8,500 and 17,600 Americans had died from H1N1 infection, with a disproportionate number of deaths occurring among healthy children and young adults. An estimated 15–25% of the nation’s population was exposed to the virus.
However, production of vaccine against this aggressive new influenza strain was agonizingly slow. A total of 18 weeks passed between identification of the new virus and the start of the pandemic’s “second wave” — 26 weeks to the peak of that second wave. But the first doses of vaccine did not become available until 26 weeks after strain identification, when spread of the virus was already at its zenith. Vaccine doses sufficient to protect 50% of the US population became available at 38 weeks, and doses to protect 100% of the population were available 48 weeks after strain identification (1).
Luckily, morbidity and mortality associated with the 2009 H1N1 influenza turned out to be lower than initially feared. Nevertheless, the sudden emergence and rapid spread of the virus was reminiscent of other, more deadly influenza pandemics — and that underscored the need for a more rapid vaccine production response in the future. In the “Spanish flu” pandemic of 1918–1919, between 25% and 30% of the world population fell ill and more than 40 million died, including more than 500,000 people in the United States alone (1).
The experience of 2009–2010 highlights the primary characteristics of pandemic influenza and key challenges that it poses to vaccine producers. Seasonal influenza, the yearly flu epidemic with which we are most familiar, comes and goes with remarkable regularity. In a process known as antigenic drift, new influenza viruses steadily evolve and annually spread through populations. People typically at greatest risk from seasonal influenza are those with weakened immune systems, primarily the elderly and patients with preexisting medical conditions. Epidemiological analysis of circulating strains during the previous influenza season usually allows for reasonable predictions regarding which will become dominant the next year. Those predictions are used to guide vaccine development. After public health authorities make strain recommendations, vaccine producers have roughly six months to manufacture, test, and release their products for distribution to the public at the start of the next flu season.
Pandemics are not so predictable. These influenza viruses arise from an abrupt genetic change — an antigenic shift — away from previously circulating viruses. As in 2009, they may arise and spread outside the normal influenza season (which is October–March in the northern hemisphere and April–September in the southern hemisphere). To make matters worse, a sudden antigenic shift away from previously circulating viruses produces a virus to which fewer members of the population have preexisting immunity. This explains the rapid spread of a pandemic strain and the particular vulnerability of children and young adults, who are less likely to have been exposed to a similar virus in their lifetime than are older adults.
In a more extreme case, influenza subtypes not normally circulating among humans — such as avian-derived H5N1 viruses — may infect humans. The complete absence of immunological memory against such viruses makes them particularly deadly and complicates the potential effectiveness of vaccines against them. Because seasonal influenza viruses steadily evolve, most individuals have some immunological memory against a new strain. Therefore, most recipients can generate neutralizing antibodies after a single dose of the seasonal vaccine without an adjuvant to boost their immune response.
Again luckily, vaccines against the 2009–2010 H1N1 pandemic influenza proved efficacious after a single immunization without adjuvant. However, those against avian-derived H5 viruses appear to require two immunizations and the inclusion of an adjuvant to elicit an effective immune response in humans. Therefore, in addition to the unpredictability and rapid spread of pandemic influenzas, producers also must contend with the possibility that such viruses may require a modified vaccine formulation and/or multiple doses to be protective.
Current State of Influenza Vaccine Manufacture
In the wake of the 2009–2010 H1N1 pandemic, US President Barack Obama ordered a review of the nation’s response to it, with specific focus on shortcomings in vaccine production and recommendations for reducing the time to produce vaccine for the next influenza pandemic that occurs. This review was conducted by the President’s Council of Advisors for Science and Technology (PCAST), and it included infectious disease and vaccine experts from academia, industry, and nongovernmental organizations. PCAST concluded that the primary factor slowing manufacture of vaccines against pandemic influenza is in the technologies used to produce them: “Fault was not with the execution of the response, but in inherent shortcomings of current technologies for development and production of influenza vaccines” (1).
All influenza vaccines currently licensed for sale in the United States are made by propagating influenza viruses in embryonated chicken eggs. As epidemiological data on emerging seasonal influenza strains are analyzed and likely strain choices are narrowed, vaccine producers select reassortant influenza virus variants for their ability to grow efficiently in eggs. After public health authorities (e.g., the FDA in the United States) make recommendations for the next season’s strain composition, producers have roughly six months to produce, test, and release their vaccines.
The system of annual surveillance, strain selection, and vaccine production has been in place for over 50 years. It relies heavily on the predictable nature of seasonal influenza, which is driven by the normally steady antigenic drift of seasonal viruses. However, because the sudden emergence of a pandemic influenza virus does not conform to egg-based vaccine producers’ timelines and capabilities, it exposes shortcomings of their decades-old technology:
Abrupt emergence and rapid spread of a new virus negates the normal lead time available for selection of egg-adapted, high-yielding vaccine strain variants. This results in a delayed vaccine production response.
Production speed is limited by the rate of virus growth in eggs.
Production capacity and flexibility are limited by egg supply and availability of specialized production facilities to accommodate
KEY BENEFITS OF RECOMBINANT VACCINES
The key benefits of recombinant vaccine production over egg-based production are faster response times and production speeds, higher surge capacities, and platform flexibility to accommodate future technological advancement:
Faster Response Time: Changing a recombinant vaccine to match a new influenza virus involves straightforward cloning of a new influenza antigen, enabling the producer to adapt quickly to a new pandemic.
Speed of Production: Recombinant technologies are not limited by the growth rate of the influenza virus in eggs or cell culture.
Surge Capacity: Use of nonspecialized production facilities enables expansion of production into alternate facilities in response to a pandemic.
Platform Flexibility: Applicability of recombinant production platforms to other vaccines or biologicals is an economic incentive for development and adoption of new technologies.
Future Technological Advances: Recombinant influenza vaccine production is expected to benefit from future advances in recombinant production technologies. For example, improvements in expression vectors, cell lines, fermentation technologies, and downstream processing should benefit the production of recombinant influenza vaccines just as they benefit other recombinant biologicals.
the biosafety classification of the virus. The potential vulnerability of chicken flocks to influenza virus represents a further supply chain risk.
PCAST recommended short-term improvements in influenza surveillance, vaccine virus development, release-testing, and fill–finish improvements to get marginal reductions in vaccine response time from the current production system. Production of influenza virus in mammalian cell culture could simplify preparedness and production but also suffers from the need to develop vaccine seed viruses, and production start-up costs would be prohibitively high. For substantial, long-term improvements in vaccine production response time and reliability, PCAST recommended fundamental changes in the technology used to make influenza vaccines: “The greatest potential for substantially shortening the time and increasing the reliability of influenza vaccine production lies in the use of recombinant DNA technologies” (1). Recombinant technology is an option with high potential to address these problems, as described in the “Key Benefits” box.
Balancing Considerations
However, recombinant pandemic influenza vaccine production faces a number of hurdles to development and implementation. The primary challenge is economic. With decades in the marketplace using the same technology, profit margins for influenza vaccines are low, and the annual “flu shot” has become something akin to a commodity. Because this low-margin market does little to encourage innovation, the federal government is providing incentives instead. In 2009, the US Department of Health and Human Services awarded a major contract to Protein Sciences Corporation for development of recombinant influenza vaccines. That was followed by additional awards in 2011 to Novavax Inc. and VaxInnate Corporation.
However, regardless of incentives for start-up and implementation, cost of goods will remain a key driver in the influenza vaccine market. New technologies will have to satisfy the requirement for high-volume and low-cost production. Platform flexibility and use of nonspecialized facilities are key features to lower the economic hurdles for companies interested in recombinant pandemic and seasonal influenza vaccine production. The potential to make a broad range of vaccines or biologicals using a platform technology potentially offsets the low profit margins inherent in influenza vaccines. Production in nonspecialized facilities, in particular without the need for live-virus biocontainment, lowers both start-up and operation costs.
As for other prophylactic vaccines, regulatory scrutiny of these processes will be particularly high, with a strong focus on safety. Because no recombinant or cell-culture based influenza vaccine has yet been approved for sale in the United States, regulatory uncertainty for approval of these new approaches remains high. However, use of a platform technology for multiple products offers the potential to develop an expanding base of knowledge and safety data to help with regulatory approvals.
Recombinant Influenza Vaccines: Current State of Development
Table 1 briefly summarizes some recombinant influenza vaccines and technologies currently under development. The list depicts a broad range of production systems and immunological mechanisms. The most advanced candidates (FluBlok and PanBlok vaccines from Protein Sciences Corporation) contain hemagglutinin, the active ingredient of currently licensed influenza vaccines, produced in insect cells (2). An earlier stage VLP vaccine from Novavax Inc. includes the influenza neuraminidase and M1 proteins in addition to hemagglutinin (3). Candidates from VaxInnate Corporation physically link the hemagglutinin antigen with flagellin, an immunostimulatory molecule (4). In other approaches, hemagglutinin or other antigens are delivered by a modified recombinant viral vector (Vaxin) or a plasmid DNA vector (PowderMed/Pfizer) (5,6). Use of alternative influenza antigens is less advanced, primarily because such vaccines reduce disease morbidity but do not prevent infection. However, interest in so-called “universal” antigens, such as the influenza A M2e peptide (constant for all influenza strains) remains high for the potential to easily stockpile such vaccines for pandemic preparedness (7). That approach has been tested by a number of producers, including Acambis/Sanofi Pasteur and VaxInnate.
Table 1: Some recombinant influenza vaccines in development

Table 1: Some recombinant influenza vaccines in development ()
Case Study: Of the recombinant influenza vaccines for seasonal or pandemic use currently in clinical development, the most advanced is a trivalent seasonal influenza vaccine (called FluBlok) under development by Protein Sciences Corporation based in Meriden, CT. This product has completed clinical trials, and a BLA has been filed and is currently awaiting FDA approval (8). My company’s PanBlok candidate is a monovalent pandemic influenza vaccine currently in clinical trials. The antigenic component of both products is highly purified, full-length influenza hemagglutinin produced in insect cell culture by recombinant baculoviruses (2). Hemagglutinin is the primary antigenic component of all currently licensed influenza vaccines. Whereas the FluBlok version is nonadjuvanted, the PanBlok product may be formulated with glucopyranosyl lipid adjuvant in a stable emulsion (GLA-SE), an adjuvant with TLR4-stimulatory properties (9).
Production of both vaccines involves standard cloning, cell culture, and purification procedures. The
hemagglutinin gene from a new influenza virus is cloned directly into a baculovirus expression vector. The qualified insect cell line (PSC uses the proprietary expresSF+ cell line) remains constant for all vaccine production. The recombinant baculovirus is then used to infect insect cells in large-scale culture; to date, baculovirus-based protein expression has been conducted in standard bioreactors up to 2,000 L. Following protein expression, infected cells are harvested using centrifugation and detergent-extracted. Hemagglutinin is purified by two column-chromatography steps, followed by DNA removal, ultrafiltration, and formulation. Because baculoviruses infect only Lepidopteran insect cells, there is no need for specialized biocontainment facilities.
As Figure 1 shows, production of the recombinant hemagglutinin antigen (rHA) contained in both FluBlok and PanBlok vaccines closely resembles mammalian cell protein production and shares many of the same unit operations. So this production can be scaled up and transferred to almost any existing mammalian cell production facility, giving it the advantages of high surge capacity, relatively low start-up and operating costs, and the potential for streamlined regulatory approval of preexisting facilities. Those features ease potential transfer of production to overseas facilities. By contrast, egg-based production of live influenza virus uses highly specialized facilities and operations in which individual eggs are infected before harvest, inactivation, and purification of virus components, then formulation into vaccine. Large-scale biocontainment facilities are required for processing tens of millions of influenza-infected eggs required for commercial vaccine production.
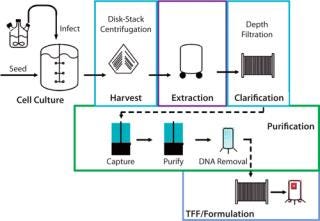
Figure 1: ()
PanBlok production response time is significantly shorter than that for egg-based vaccine production. Figure 2 shows the timeline for PanBlok clinical production in response to the 2009 H1N1 outbreak (8). Day 0 represents receipt of the H1N1 vaccine virus candidate from the CDC. Cloning into the baculovirus expression vector, baculovirus plaque isolation and virus expansion took 30 days, culminating with freeze-down of a qualified working virus bank. Scale-up manufacturing began 45 days after receipt of the H1N1 virus sample, and vaccine release testing was complete after 75 days for initiation of a pandemic influenza clinical study. The 11-week timeline from the start of cloning to vaccine release represents a significant reduction from vaccine production time of commercially released H1N1 vaccines in 2009, which took 26 weeks for the first doses to become available. With the second wave of the 2009 pandemic beginning 18 weeks after identification of the H1N1 virus and peaking at 26 weeks, vaccine release in
Figure 2: ()
This article is adapted from a presentation given at Interphex in New York, NY, March 2011 in a lunchtime session moderated by BPI’s editor in chief, S. Anne Montgomery.
About the Author
Author Details
Albert Price, PhD, is technical director for influenza at Protein Sciences Corporation, 1000 Research Parkway, Meriden, CT 06450; 1-203-686-0800, x367; fax 1-203-686-0268; [email protected]
REFERENCES
1.) 2011.President’s Council of Advisors on Science and Technology Report to the President on Reengineering the Influenza Vaccine Production Enterprise to Meet the Challenges of Pandemic Influenza, Executive Office of the President, Washington.
2.) Wang, K. 2006. Expression and Purification of an Influenza Hemagglutinin: One Step Closer to a Recombinant Protein-Based Influenza Vaccine. Vaccine 24:2176-2185.
3.) Ross, TM. 2009. A Trivalent Virus-Like Particle Vaccine Elicits Protective Immune Responses Against Seasonal Influenza Strains in Mice and Ferrets. PLoS ONE 4:e6032.
4.) Song, L. 2008. Efficacious Recombinant Influenza Vaccines Produced By High Yield Bacterial Expression: A Solution to Global Pandemic and Seasonal Needs. PLoS One 3:e2257.
5.) van Kampen, KR. 2005. Safety and Immunogenicity of Adenovirus-Vectored Nasal and Epicutaneous Influenza Vaccines in Humans. Vaccine 23:1029-1036.
6.) Jones, S. 2009. DNA Vaccination Protects Against an Influenza Challenge in a Double-Blind Randomized Placebo-Controlled Phase 1b Clinical Trial. Vaccine 27:2506-2512.
7.) Fiers, W. 2009. M2e-Based Universal Influenza A Vaccine. Vaccine 27:6280-6283.
8.) Cox, MJ, and Y Hashimoto. 2011.A Fast Track Influenza Virus Vaccine Produced in Insect Cells J. Invertebrate Pathol.
9.) Coler, RN 2010. A Synthetic Adjuvant to Enhance and Expand Immune Responses to Influenza Vaccines. PLoS One 5:e13677.
You May Also Like






