Tunable Half-Life TechnologyTunable Half-Life Technology
March 1, 2013
While a constantly developing market puts increasing pressure on pharmaceutical companies to provide advanced and personalized therapies, the industry is investing heavily in the development of targeted biologics. The aim is often to take new therapeutics through clinical trials and to market as quickly as possible and to develop more novel, tailored drugs. One common challenge for many biologics is their short plasma half-life. That often leads to reduced bioavailability, meaning that an administered drug will clear from a patient’s system within a matter of minutes. So patients with chronic conditions need regular high dosages, which increases costs to patients and the likelihood of side effects. Frequent administration of a drug may also decrease patient compliance with treatment regimes.
Addressing the half-life challenge has been a focus of industry product development in recent years. Real progress has been seen in development of technologies to modulate the serum half-lives of protein-based therapeutics to meet desired specifications. Increasing hydrophobic volume (through PEGylation, fusing proteins to polyethylene glycol molecules) and FcRn-mediated recycling (albumin fusion) are common strategies. Although both have been successful in extending serum half-life, researchers continue to work on specifically designing protein half-life to achieve desired pharmacokinetics.
PRODUCT FOCUS: BIOLOGICALS AND SMALL MOLECULES
PROCESS FOCUS: MANUFACTURING
WHO SHOULD READ: PRODUCT AND PROCESS DEVELOPMENT, ANALYSTS, AND FORMULATORS
KEYWORDS: ALBUMIN, FUSION, CONJUGATION, DRUG DELIVERY, EXPRESSION/PRODUCTION
LEVEL: INTERMEDIATE
Novozymes has developed a half-life extension technology to offer a flexible drug delivery platform. It is based on the natural, nonimmunogenic plasma protein (albumin), which is well known for its safe and proven regulatory profile. Using this new tool, manufacturers can modify protein or peptide half-life to suit the function of a specific drug. It also helps researchers manage and preserve efficacy while defining the pharmacokinetics of their target proteins.
Advancing Albumin Fusion Technology
Most protein therapeutics display a half-life measured in minutes, but serum albumin takes a 19 days to clear from a human system. That long half-life is attributable to both the molecule’s size and pH-dependent recycling through neonatal FcRn receptors, both of which protect albumin from renal clearance. Like immunoglobulin G antibodies (IgGs), albumin is taken up by cells through nonspecific pinocytosis. Inside cells, it is protected from intracellular degradation through pH-dependent binding to the FcRn in acidic endosomes. That FcRn interaction allows albumin to then recycle back to a cell’s surface, where it is released back into circulation because of the physiological pH of blood (Figure 1).
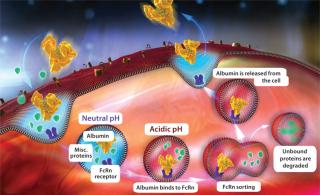
Figure 1: ()
Recent developments in albumin fusion technology are based on the pH-dependent interaction between albumin and FcRn. Understanding that relationship and its impact on albumin fusion half-life has enabled engineering of such an interaction to modulate albumin’s half-life. Previous studies showed that altering the interaction between IgG and FcRn affected pharmacokinetics of IgG (1). By understanding and implementing those kinetic principles, Novozymes’ new technology can modulate protein half-life through construction of albumin variants that have altered binding affinity to FcRn.
The University of Oslo in Norway worked alongside Novozymes to identify amino acids that may be involved in binding albumin to FcRn (2). Research has involved cross-species binding studies as well as analysis of polymorphisms and sequence alignments. Subsequently, numerous albumin variants were generated with single amino-acid substitutions. Binding-affinity studies of each variant to FcRn at acidic pH helped identify single amino-acid positions that can generate a range of affinity variants with distinct binding differences. Those variants were measured with both increased and decreased receptor binding affinities, which could translate to modulation of albumin half-life.
With that ability to modify albumin half-life, protein developers get the chance to fine-tune their drug design. In addition to the more common application extending protein half-lives, the degree of protein half-life extension can be reduced for highly toxic drugs (e.g., antibody–drug conjugates).
A New Approach
The new half-life extension technology can increase a protein’s half-life from hours to days — and from days to weeks. So a drug can be formulated for reduced dosing frequency and improved performance through an increased half-life. Such half-life flexibility allows manufacturers to tailor treatment regimens and target innovative drugs to the particular needs of patients who suffer from chronic or acute conditions such as diabetes, hemophilia, and neutropenia.
The limited number of biologics on the market limits the choices that pharmaceutical companies have. Manufacturers are looking to adjust and develop available molecules, and this technology will give them the opportunity to cultivate niche market positions in the market and provide novel and flexible products. From both patient and commercial perspectives, the innovation affords companies a definite competitive advantage in improving the lives of patients who suffer from chronic illnesses. The technology could decrease the frequency of and lower dosage levels for patients who need to take regular medication, encouraging patient compliance and even making it possible for some patients to self-administer their own medications.
Drug Delivery Methods
Conjugation and fusion technology (Table 1) can both be used with half-life extension technology, depending on specific drug-delivery requirements. Lysine, tyrosine, and free thiol residues of the albumin molecule are used to chemically conjugate it to a drug product. The most widely used route involves a free thiol at albumin position 34. That approach is particularly useful for peptides that contain maleimide groups, which specifically react with the free thiol to form a stable thioether bond. Alternatively, proteins can be genetically fused to the N- or C-terminus or even to both ends of the albumin variant. Using a contiguous complementary deoxyribunucleic acid (cDNA) of the target protein or peptide with DNA encoding a chosen albumin variant allows generation of protein fusions that exhibit required binding characteristics. A yeast expression system provides a high-quality, consistent, and reliable supply of the protein of interest with a genetic fusion applied.
Table 1: Comparing albumin conjugation and genetic fusion strategies
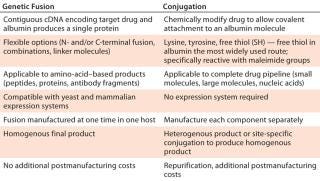
Table 1: Comparing albumin conjugation and genetic fusion strategies ()
Several albumin protein fusions have been generated to determine whether the albumin variants maintain their modified FcRn binding affinity when fused to proteins or peptides. The variants tested displayed a range of binding affinities from low-affinity albumins (HSA K500A) to those with a 15-fold increase in receptor binding (HSA K573P). Figure 2 reports on comparisons of antibody fragments fused at the C- or N-terminus, as well as bivalent forms and fusions to small or large peptides, with unfused albumin variants for FcRn affinity using Biacore surface-plasmon resonance technology. Each albumin fusion presented different receptor affinities, correlating to its unfused variants. And each showed the same differences in ScRn binding as the control rHSA variant.
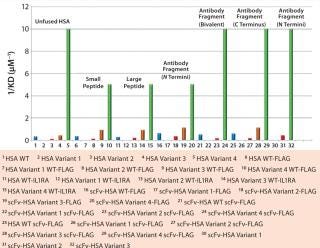
Figure 2: ()
Using this flexible technology, proteins and peptides can bind at either the C- or N- terminus or both to create fusion molecules with monovalent, bivalent, or bispecific affinities. In addition to protein- and peptide-based drugs, this technology can serve as a delivery vehicle for small molecules. It also enables construction of albumin variants with altered binding affinities to FcRn, making it possible to modulate the half-life extension of fused target proteins and offering drug developers enhanced flexibility and control over dosage regimens.
Better for Manufacturers, Better for Patients
Market demand for more effective drugs is growing, which has led to recent development of a range of half-life extension platforms. Many such platforms are limited by the synthetic materials from which they are developed; that is, drug half-life cannot be effectively adapted to fit the needs of specific medical conditions. In response to that challenge, the latest advancement provides a wide-ranging and accessible platform that drug manufacturers can use to differentiate their own products from competing therapies — as in creating “biobetter” products. Thanks to new knowledge of albumin’s relationship with its receptors, this technology could transform many products.
Following the launch of this half-life extension technology to market, pharmaceutical companies of all sizes have begun to draw on its benefits — e.g., some using the technology for diabetes, hemophilia, and neutropenia treatments. Because this technology is based on albumin (a natural, nonimmunogenic plasma protein already present in our bodies), it is safe for use in pharmaceutical development to help provide control and design flexibility. Reduced dose frequencies can offer patients more effective treatment plans and thus a higher quality of life.
About the Author
Author Details
Mark Perkins, PhD, is a customer solution manager at Novozymes Biopharma UK Ltd., 59 Castle Boulevard, Nottingham NG7 1FD, UK; 44-115-955-3355; [email protected]; www.novozymes.com. For further information on the half-life extension technology described herein, see www.daystoweeks.com.
REFERENCES
1.) Suzuki, T. 2010. Importance of Neonatal FcR in Regulating the Serum Half-Life of Therapeutic Proteins Containing the Fc Domain of Human IgG1: A Comparative Study of the Affinity of Monoclonal Antibodies and Fc-Fusion Proteins to Human Neonatal FcR. J. Immunol 184:1968-1976.
2.) Anderson, JT. 2012. Structure-Based Mutagenesis Reveals the Albumin-Binding Site of the Neonatal Fc Receptor. Nature Commun 3:610.
You May Also Like






