Travel in January turned out to be more of an adventure than many of us anticipated. Two events that BPI attends in January were in Washington, DC: Phacilitate’s cell and gene therapy conference (part of its Washington Leaders Forum) and the CASSS Well Characterized Biotechnology Products conference (along with two CMC strategy forums). These two events present topics that we see carried throughout the year, including FDA initiatives and current regulatory, quality, analytical, and manufacturing focus points.
Organizers of both events were expecting record participation — and then came winter storm Jonas. Such conditions are outside my experience as an Oregonian; just a light dusting of snow in our valley is cause for school cancellations and road trouble. But I was impressed by the schedule adjustments throughout that week. Both conferences’ organizers canceled Monday programming and moved what sessions they could into the remaining days.
The Phacilitate Bioleaders Conference still drew about 400 people,...
Introducing a New Editorial Advisor
Nanda Subbarao
is a senior consultant with the Biologics Consulting Group specializing in analytical, stability, and quality systems for chemistry, manufacturing, and controls (CMC) with good laboratory and manufacturing compliance (GLPs, GMPs). She has assisted pharmaceutical and biotechnology organizations in evaluation of analytical methods and method validation for a broad range of products ranging from conventional drugs to well-characterized proteins and vaccines, from preclinical to commercial phases. Subbarao has been involved several times over her career in projects to set up or upgrade current GMP- (CGMP) and GLP-compliant quality systems for laboratory and stability programs for products in development and on the market. She also participates in the AAPS Stability Focus Group Steering Committee.
Previously, Subbarao worked as stability manager for Sandoz (Novartis) in Dayton, NJ, where she led a group of chemists and sample coordinators/stability administra...
WWW.GRAPHICSTOCK.COM
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity.
In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its
GMP Data Integrity Definitions and Guidance for Industry
, stating, “Data integrity is fundamental in a pharmaceutical quality system, which ensures that medicines are of the required quality” (
1
). This fundamental concept should not be taken lightly because consequences can be severe, but it can often be a challenge for organizations to successfully implement.
Here I outline basic expectations surrounding data integrity and the strategic, multitiered approach an organization can implement to ensure that its p...
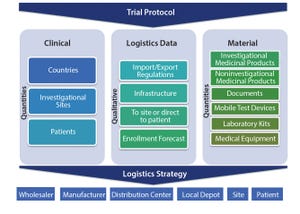
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (
1
). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers.
The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application materials (e.g., infusion pumps and some medicinal products and biological samples) may need to be stored in refrigerated conditions.
In most clinical studies, the majority of data is analyzed using patients’ laboratory samples. Those samples are collected in syringes, blood-collection tubes, urine-specimen containers, and so on. Patient blood samples drawn in special tubes may need to be centr...
Biological additives such as yeast extracts and peptones are commonly used in growth-media formulations for biopharmaceutical manufacturing. In spite of drivers encouraging companies to reduce variability in mammalian cell culture processes by using chemically defined media, many microbial and mammalian processes continue to use biological additives in their growth-medium formulations and/or feeds. According to Sheffield Bioscience (Kerry, Inc.), at least six of the top 10 licensed mammalian-cell– derived biotherapeutic products are manufactured using biological additives (
1
).
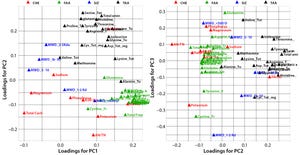
During process development, it can be difficult to identify which of the biological additives will be appropriate for a particular application because of their complex composition and the number of biological additives available. Multivariate analysis (MVA) techniques are increasingly used to help companies understand the complexity of their biopharmaceutical products and associated manufacturing processes (
2
). Techniques includ...
WWW.PHOTOS.COM
Many biotherapeutic proteins are naturally subject to aggregation. The clinical consequences of protein aggregation can be dramatic, not only affecting bioavailability and pharmacokinetics, but in extreme cases dramatically altering pharmacodynamics as well. Of equal or perhaps more importance is that aggregation is a principal source of unwanted immunogenicity in biotherapeutics. Aggregation-induced neutralizing antibodies and/or anaphylactic reactions are serious and growing US and European regulatory concerns. So they will have significant and growing influence on the future development and regulatory approval of both biosimilar and innovator biotherapeutics.
Surfactants are routinely used to prevent or limit protein aggregation. The most commonly used surfactants for this purpose are Tween 80 and Tween 20 polysorbates. They are both effective in preventing aggregation, but polysorbates are known to spontaneously oxidize to chemically reactive peroxides, epoxy acids, and aldehydes that c...
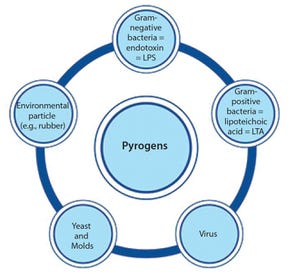
Figure 1: Pyrogens constitute a diversive heterogenous group of contaminants comprising microbial and nonmicrobial substances.
In the early 20th century, some patients injected with the drug Salvarsan experienced febrile reactions due to contamination of the drug’s distilled water. That incident (involving the first effective treatment for syphilis) prompted not only the widespread use of injectable drugs, but also the need for pyrogen control. Pyrogens constitute a heterogeneous group of microbial and nonmicrobial substances that include those derived from Gram-negative and Gram-positive bacteria such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), respectively, as well as particles from yeasts and viruses (Figure 1). When present as contaminants in pharmaceutical products, pyrogens can activate the human immune system and induce life-threatening fever reactions.
The response of the human immune system to pyrogens is extremely fine-tuned, which explains the unusually consistent reaction among in...
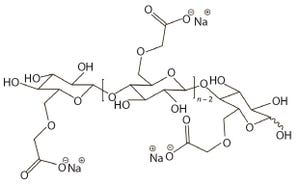
Figure 1: Idealized possible unit structure of sodium carboxymethylcellulose (CMC) with a DS of 1.0; the DS would be 3.0 if all three hydroxyl groups on anhydroglucose unit were substituted. A DS of 3.0 is the theoretical maximum for CMC.
Carboxymethylcellulose sodium (CMC), is widely used as an excipient in oral, topical, and parenteral pharmaceutical formulations. It increases viscosity (
1
–
3
), serves as a suspension aid (
4
), and stabilizes emulsions (
5
). More recently, applications for CMC in formulations that facilitate improved delivery of cytotoxic drugs and biologics have been evaluated (
6
,
7
).
CMC is manufactured in a broad range of viscosities, with grades typically classified as low, medium, or high viscosity. CMC grades can be divided further based on their degree of substitution (DS), which is defined as the average number of hydroxyl groups substituted per anhydroglucose unit (Figure 1). Together, DS and the extent to which carboxymethyl substituents cluster determine functional pr...
Insulin injection
Discovered in 1922 at the University of Toronto (by Frederick Banting and John James Rickard Macleod), insulin has been produced from animal extract, primarily cattle and pigs. Following Frederick Sanger’s work on insulin sequence in 1951, Dorothy Hogkin published in 1969 the three-dimensional structure of insulin. This breakthrough led to many developments and applications of recombinant DNA for the production of insulin, human first and then analogs.
The significant increase in the diabetic population — especially in BRICS countries (Brazil, Russia, India, China, and South Africa) — has pushed local pharmaceutical manufacturers to focus on this major health issue. These nations are developing either local production capacities or an adequate supply chain to answer domestic demands. But insulin production can bring many difficulties and requires robust processes to ensure the safety and effectiveness of final products.
Herein we discuss how the engineering company Biopharmax established...
Olympics fans aren’t the only ones turning their eyes toward Brazil. According to JLL’s fourth annual
Life Sciences Outlook
, the 2016 Summer Games host nation is also one of the world’s top 10 “Global Clusters to Watch,” thanks to its proven capacity for medical device manufacturing and a growing healthcare market. Increasingly, Latin America overall has been on the radar for life sciences companies seeking greater operational efficiency and opportunity, expanded market access, promising demographics, lower-cost land and labor, and emerging pathways to innovation.
National governments across Latin America have improved their stability and are entering into liberal trade agreements. With increasing support for healthcare and growing middle class populations in the region, consumer demands for drugs and treatments also are expanding. One report projects healthcare spending across Latin America to grow on average 4.6% annually through 2018 (
1
). Talent pools also are expanding as more citizens gain access...