Every September, we editors step onto a virtual slide that takes us into the new year with increasing steepness and velocity. Here come the fall supplements and special projects! And as the new year approaches, our momentum is often halted abruptly by the November and December holiday schedules, during which many of our contacts seem to vanish.
The highlight of our fall season is always the
BioProcess International Conference and Exposition
in Boston, this year occurring the very week that you should be receiving this issue and supplement. We work closely with our Informa conference colleagues to ensure that conference presentations and print offerings together enhance your work experience, providing multiple viewpoints on key themes. We collaborate for on-site interviews and webcasts, preconference white papers, and other highlights. And we seek to develop manuscripts with those presenters whose especially timely talks generate the greatest “buzz” during our week in Massachusetts.
We hope to see you th...
Adaptive Pathways Pilot Program Results
A final report became available this past summer about the European Medicines Agency’s (EMA) pilot project on developing medicines for unmet needs. The project showed that adaptive pathways can bring multiple stakeholders together — including regulators, health-technology assessment (HTA) bodies, healthcare professionals, and patients — to evaluate drug development and data gathering.
Adaptive pathways allow for a planned, progressive approach to bringing medicines to patients. The concept makes use of existing approval tools (e.g., conditional market authorization) used in the European Union since 2006. It also builds on experience gained with strengthened postmarket monitoring introduced by the European Union’s 2012 pharmacovigilance legislation. Under this paradigm, drugs are authorized for small groups of patients who benefit most from them. Then as additional evidence is gathered on each drug’s performance, progressive licensing adaptations will extend or restr...
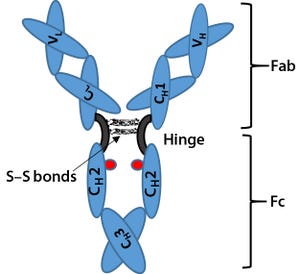
Figure 1: Immunoglobulin G (IgG) structure consists of four polypeptide chains, including two identical light chains (~25 kDa) and two identical heavy chains (~50 kDa). Each light chain consists of one constant domain (C
L
) and one variable domain (V
L
), and each heavy chain consists of three constant domains (C
H
1, C
H
2, and C
H
3) and one variable domain (V
H
). The Fab is the antigenbinding region containing hypervariable or complementarity-determining regions (CDRs), whereas the Fc region is highly conserved across molecular species. The hinge region contains two disulfide bonds and glycosylation (shown as red dots) at C
H
2 domain of Fc fragment.
Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structu...
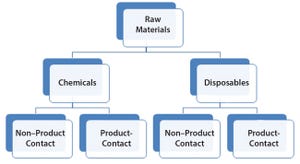
Figure 1: Raw materials high-level classification
Ensuring a continuous supply of safe medicines to patients is a key objective for both health authorities and the pharmaceutical industry. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs). Manufacturers must ensure not only the suitability of RMs for their intended use in a manufacturing process, but also their highest attainable safety with regards to viruses and other adventitious agents. The need to apply a risk-based RM control strategy is in line with regulatory expectations and is recognized within the biomanufacturing industry (
1
–
3
).
Viral contamination is a potential safety threat common to all animal- and human-derived biologics produced in mammalian cell culture (
4
). A viral contamination can arise from a contaminated cell line source or from adventitious introduction of virus during production. Although contamination of bioreactors and biologic therapeutics is rare, several manufacturers ha...
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules such as monoclonal antibodies (MAbs). That strategy includes identifying a standard set of raw materials, process parameters, and analytical methods (a control strategy) for the platform manufacturing strategy. Such a platform is well established at Cook Pharmica, where the process development team continues to evaluate new process technologies to assess them for fit and help the company remain flexible in our offerings. Here we share some data generated with an IgG1 at Cook ...
Along with increased selectivity, mixed-mode and multimode media offer significant potential operational cost savings by eliminating intermediate purification steps that require additional time, materials, equipment, and personnel.
Continuing development in protein and peptide engineering have produced a broad range of new biological products with improved therapeutic and diagnostic potential. In the development pipeline, more than 900 biologic products target more than 100 diseases (
1
). Increased manufacturing complexities caused by closely related impurities and requirements to improve process efficiencies and reduce operating costs highlight the need for new approaches in protein purification.
Platform-based chromatographic approaches have been successfully applied in separating and purifying monoclonal antibody (MAb) products. But the next generation of therapeutic proteins — e.g., tetravalent bispecific antibodies, bispecific T-cell engagers (BiTes), nanobodies, and antibody fragments — presents di...
A researcher at MilliporeSigma, the life science business of Merck KGaA, Darmstadt, Germany, investigates the ability of tentacle ion-exchange media to remove viruses effectively in monoclonal antibody purification processes.
Manufacturers strive toward cost-effective purification of target molecules and a high level of confidence that their biologics are safe and not compromised by the presence of endogenous retrovirus-like particles or adventitious viruses (
1
). Reliable reduction of viral particles throughout downstream purification processes must be ensured through different techniques such as chemical treatment, filtration, and chromatography. Common monoclonal antibody (MAb) purification schemes use both cation- and anion-exchange chromatography steps (CEX, AEX). Although CEX (to remove product- and process-related impurities) is not generally considered robust enough for virus reduction, in some conditions it has made a significant contribution to virus removal. By contrast, AEX (a polishing step ...
Photo 1: Standard one-bottle position in chamber
Freeze–thaw processes affect the quality of biopharmaceutical proteins (
1
–
13
) and human cells (
14
). It has been reported that no method consistently controls freezing and thawing rates for biological formulations (
1
). My recent study refutes that claim with validated rate-controlled freezing and thawing of such formulations in 16-L single-use bags (
15
).
The study reported herein presents a consistent method for controlled-rate freezing and thawing of bottled formulations. It also highlights the effect of load and container position on freeze rates. The freeze–thaw controlled-rate chamber used in this study is a Model 4002 manufactured by Farrar Scientific. It permits rapid, uniform bulk freezing/ thawing of products with temperature ranges from +40 °C to –80 °C. The model has a minimum of 1.7 kW of net cooling and heating capacity over its entire temperature range. Units come equipped with programmable profiles that allow flexibility to meet uniqu...
A new report from Thomson Reuters shows that innovation in biotechnology declined slightly in 2015, and biotech is the only one of a dozen worldwide industries examined to show that kind of decline (
1
). To measure innovation, compilers used metrics such as patents filed and scientific literature cited. Looking at the details, however, the dip was just a 2% drop from 42,584 events in 2014 to 41,624 in 2015. The same dynamics had revealed a 7% increase in innovation from 2013 to 2014.
Indeed, the report notes that the sector experienced a number of “firsts” in 2015. It cites as examples both CRISPRs (clustered regularly interspaced short palindromic repeats, segments of prokaryotic DNA) and editing of a human-embryo germline. “CRISPR ‘interference’ involved making targeted modifications to segments of DNA to alter its immunity,” the report states. “Such work has implications not only for humans, but also for food crops and other plants and animals.”
Other noteworthy innovations highlighted in the report i...
Acceleration of Bioprocess Development Using DoE and Physiological Control
with Christoph Herwig
Understanding the interdependencies among process variables, product quality attributes, and process parameters is key to achieving quality by design (QbD). Developers need to gather as much meaningful information as possible from their experiments. In a BPI “Ask the Expert” webinar on 7 August 2016, Professor Christoph Herwig of the Vienna University of Technology demonstrated how experiments based on physiological key parameters can be linked to design of experiment (DoE) approaches to accelerate bioprocess development. Herwig recommended an all-in-one tool for such work: the Lucullus process information and management system from Applikon.
Herwig’s Presentation As a unique tool for bioprocess development, piloting, and manufacturing, Lucullus software has four main features for planning, preparation, execution, and evaluation of experiments. Herwig focused on the latter. “These elements can be done not only...
Modification of Glycans in Bioprocessing
with Ryan Boniface
Protein quality determines clinical behavior. Glycosylation is a key product quality attribute for many biotherapeutic proteins expressed by mammalian cells. N-linked glycans can display macro- and microheterogeneity with a degree of variation that depends on several factors. It’s often challenging to predict, achieve, and maintain preferred glycosylation profiles. For an “Ask the Expert” webinar on 15 June 2016, bioproduction scientist Ryan Boniface of Thermo Fisher Scientific explored approaches to modifying glycan patterns.
Boniface’s Presentation
Thermo Fisher is strongly focused on enhancing protein quality both through cell culture process and media. The glycosylation profile of a protein is an important parameter that affects its structural and functional characteristics as well as in vivo half-life, efficacy, metabolic activity, and clearance rates.
Cell growth and product titer don’t matter if expressed product is low in quality. Widely ...