January-February 2021 Featured Report
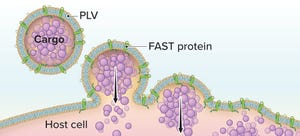
Figure 1: The Fusogenix proteolipid vehicle (PLV) platform; fusion-associated small transmembrane (FAST) proteins catalyze rapid lipid mixing between a PLV and a target cell’s plasma membrane. PLV cargo is deposited directly into the cytoplasm, bypassing the endocytic pathway.
Although vaccine platforms based on messenger RNA (mRNA) are enjoying the limelight in the wake of emergency authorizations of products from Pfizer–BioNTech and Moderna, DNA vaccines are poised to make their own commercial debuts soon. The World Health Organization (WHO) reports that six of the 48 candidate vaccines against SARS-CoV-2 that remain in clinical trials are DNA-based products, as are 14 others in preclinical study (
1
).
I spoke with Hong Jiang (cofounder and chief operating officer of Aegis Life, Inc.) in November 2020 to learn more about plasmid-DNA vaccines. Aegis Life spun out of Canadian biopharmaceutical company Entos Pharmaceuticals early in 2020 to pursue US-based research and development (R&D) of a pancoronaviru...
Arktek cryostorage containers holding Ebola vaccines for distribution in Sierra Leone; in 2015, cold-chain engineering company Modality Solutions supported clinical trials for Ebola vaccines in that country.
(Photo Courtesy of
Modality Solutions
)
In the wake of fast-track approvals for Pfizer’s and Moderna’s respective SARS-CoV-2 vaccines, now begins the largest immunization campaign in world history. Its success will depend not only on the products’ safety and efficacy, but also on several mass-distribution programs requiring significant cold-chain infrastructure. The public has become acutely aware of the Pfizer vaccine’s demanding cryostorage specifications, generating considerable anxiety about how mass distribution will happen. Behind the scenes, however, cold-chain engineering companies such as Modality Solutions have worked alongside drug developers to ensure that vaccines can be shipped efficiently without compromising drug-product safety and quality.
In December 2020, I spoke with Gary M. Hutch...
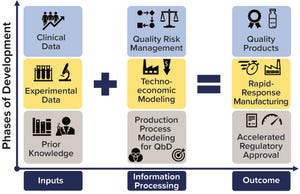
Pandemics such as the current COVID-19 outbreak pose tremendous healthcare and economic challenges. Vaccines hold promise for controlling pandemics; however, substantial challenges come with pandemic-response vaccine development, manufacturing, distribution, and administration. To address those now, many companies are using rapid-response vaccine-production platform technologies. Computational modeling tools could help further accelerate development of those technologies, increase production and distribution efficiencies, and reduce costs and risks once vaccine platforms are fully developed and validated. To those ends, a set of modeling methodologies — including bioprocess modeling for quality by design (QbD), technoeconomic, and supply chain modeling planning — can be combined with prior knowledge and current data from underlying physical processes. The results provide a framework to accompany and enhance vaccine production and distribution processes.
Figure 1: Framework for further accelerating regulat...
Biopharmaceutical companies are racing to develop vaccines that mitigate the COVID-19 pandemic, taking a wide range of vaccine-development approaches that include traditional modalities and cutting-edge technologies based on DNA and RNA. Vaccine developers are leveraging robust manufacturing concepts and integrated processes to shorten timelines. Advanced analytics also are playing a critical role in ensuring the safety and efficacy of those emerging vaccines.
A New Wave of Vaccines
Vaccines based on attenuated viruses entail development timelines ranging from four years to 15 years (
1
). Thus, despite that approach’s proven efficacy, it is inappropriate for rapid responses. A virus must be isolated from hosts, a reliable culture system must be established, and the pathogen must be weakened chemically or biologically to ensure patient safety while eliciting sufficient immune responses. Finally, adjuvants must be optimized for final dosing. All those steps require validation using several analytical techn...
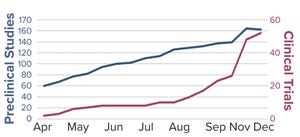
Figure 1: SARS-CoV-2 candidate vaccines in the preclinical and clinical evaluation phases
Traditionally, viruses for vaccines have been grown in embryonated hen eggs. But new challenges introduced by the COVID-19 pandemic have encouraged and catalyzed innovations in the field of vaccine development. The biopharmaceutical industry has recognized an advantage in mammalian cell-culture systems as promising alternatives to egg-based vaccine production. Cell lines can be cultured to large quantities in bioreactors, allowing for much shorter lead times, a more controlled production process, and a higher grade of reproducibility through standardization.
In this article, David Solbach explains how advancing single-use and automation technologies could accelerate cell-based vaccine development and production further. He also describes why stirred-tank bioreactors represent a key technology needed on the journey through developing and producing a new vaccine. Read on to learn how the flexibility and scalability of ...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.