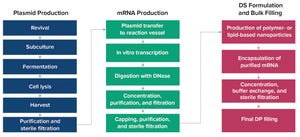
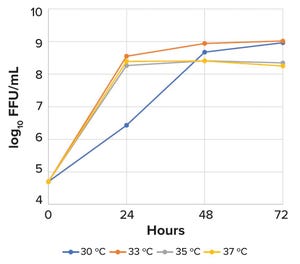
January-February 2023 Featured Report
2,000-L bioreactor and associated control system for production of viral vectors at the ReiThera facility in Rome, Italy
(
HTTPS://REITHERA.COM
)
Viral vectors continue to generate considerable excitement in the biopharmaceutical industry, albeit primarily for advanced-therapy applications. The BPI editorial team receives multiple manuscripts each year relating to production, purification, and formulation of adenoassociated virus (AAV) and lentivirus (LV) vectors for in vivo delivery of gene therapies and ex vivo modification of patient/donor cells, respectively. Compared with such applications, viral-vector vaccines receive far less attention; however, they merit serious consideration because they hold much promise for mitigating infectious diseases.
Viral-vector vaccines are not entirely new technologies. The first use of a viral vector to express a foreign gene goes back to 1972, when Jackson et al. used simian virus (SV) 40 to carry genes from enterobacteria phage λ and
Escherichia coli
(
1
). In 1...
Vaccines based on messenger ribonucleic acid (mRNA) created headlines in December 2020 for being the first highly efficacious SARS-CoV-2 prophylactics to receive emergency use authorization (EUA) from the US Food and Drug Administration (FDA). Within a couple months of the virus’s gene sequence being published (
1
), Pfizer and BioNTech were ready with their vaccine candidate (
2
), and in less than a year, the vaccine was approved for administration in adults. EUA for Moderna’s mRNA vaccine followed soon after. The rapidity with which those products were developed stands in stark contrast to the 10–15 years that traditionally have been required for a candidate vaccine to move from discovery to approval and administration in patients.
Pfizer–BioNTech’s and Moderna’s respective vaccines were developed in such short timeframes because mRNA technology was not designed overnight to mitigate COVID-19. Scientists have been studying mRNA vaccines for almost three decades to protect against influenza, rabies, Zik...
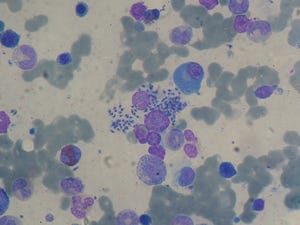
Bone marrow aspirate from a liver-transplant recipient showing infection with visceral leishmaniasis
(PAULO HENRIQUE ORLANDI MOURAO,
HTTPS://WIKIPEDIA.ORG
)
The World Health Organization (WHO) considers leishmaniasis to be one of the world’s most neglected tropical diseases (NTDs). As of January 2023, more than one billion people are at risk of infection with leishmaniasis because they live in endemic regions. NTDs disproportionately affect the world’s most underresourced and malnourished populations, contributing to a vicious cycle of poverty and disease. Yearly, between 498,000 and 862,000 new cases are diagnosed, resulting in over 18,700 deaths and up to 1.6 million disability-adjusted life years (DALYs) lost (
1
).
Leishmaniasis is a vector-borne disease caused by protozoan parasites of the genus
Leishmania
, which are transmitted through the bites of infected female sandflies and which manifest in three ways (
2
). Also known by the Hindi term
kala-azar
, meaning “black sickness,” visceral leishma...
WWW.ALAMY.COM
Liquid-formulation vaccines often require shipment and storage within a temperature-controlled supply chain (
a cold chain
) between manufacture and administration. Since 2020, mRNA vaccines against SARS-CoV-2 have received considerable attention for their extreme cold-chain specifications. For instance, Pfizer–BioNTech’s Comirnaty bivalent booster for adults and adolescents must be stored in specialized freezers set between –90 C and –60 C for as long as 18 months (
1
). Usually, however, vaccines are shipped and stored in conditions between 2 C and 8 C (the temperature range measured during accelerated and long-term product-stability studies) because of their susceptibility to degradation and aggregation upon freezing (
2
). That is particularly true for aluminum-adjuvanted products, including many vaccines that are listed on recommended pediatric inoculation schedules (
3
). Such problems can decrease vaccine potency, raise unwanted immunogenicity concerns, and compromise patient safe...
Sponsored Content
Newcastle disease is an extremely infectious condition among domesticated poultry and other avian species. Its high morbidity and mortality rates among infected birds give the disease significant economic importance. Thus, many commercially available vaccines based on live or inactivated virions are used globally to protect against Newcastle disease infection.
The causative agent is Newcastle disease virus (NDV), which belongs to the
Paramyxoviridae
family. NDV is a single-stranded, negative-sense, enveloped RNA virus of avian origin that is highly attenuated in humans and other primates because of strong host-range restriction (
1
). Attenuated NDV has been evaluated as a vector for vaccines against SARS-CoV-2 (
2–4
), Ebola (
5
), H5N1 influenza (
6
), West Nile (
7
), and simian immunodeficiency viruses (
8
). Oncolytic NDV vectors also hold much promise for immunotherapies against various cancers (
9, 10
).
Whether for vaccines or viral-vector therapies, NDV particles must meet certain criteria for ...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.