June 2018 Featured Report
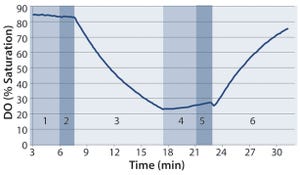
Figure 1: Example of dissolved oxygen (DO) profile over the course of one test: (1) stop rocking and compress to eliminate headspace air; (2) inflate with nitrogen; (3) start rocking; (4) stop rocking and compress to eliminate headspace nitrogen; (5) inflate with air; (6) starting rocking at predetermined test conditions
Disposable bioreactor systems are technologies commonly used in bioprocessing. They provide cost-effective contamination control and allow more flexible facility layouts than do stainless steel alternatives. One of the most popular types of single-use bioreactors uses a rocking platform in place of a traditional shaft and agitator assembly to aerate and mix cell culture material within a presterilized, single-use plastic bag (
1
). The system studied here is the ReadyToProcess WAVE 25 bioreactor (GE Healthcare Life Sciences).
In contrast to conventional stirred tanks, rocking-motion bioreactor systems do not rely on oxygen spargers for culture aeration or agitators for mixing. Instead, th...
PHOTO BY ERIC SCHOLLENBERGER
Emerging cell therapies have excited the pharmaceutical industry because they indicate potential new pathways to treat some of the most life-threatening diseases. T-cell therapies currently are the flagship technology in cell therapy with recent US FDA approvals of Novartis’ Kymriah (tisagenlecleucel) and Gilead’s Yescarta (axicabtagene ciloleucel) treatments. Those therapies and others still in development use peripheral blood isolated lymphocytes (PBLs) modified with chimeric antigen receptors (CARs) or modified T-cell receptors (TCRs) to trigger the innate cytotoxic response of these immune cells for specific tumor types (
1
,
2
). Coupled with the safety of an autologous therapy, CAR and other T-cell–based treatments serve as powerful and unique tools for treating a range of diseases in oncology and other areas of medicine.
With regulatory recognition of the efficacy of such treatments comes the realization of manufacturing hurdles that need to be resolved for large-scale...
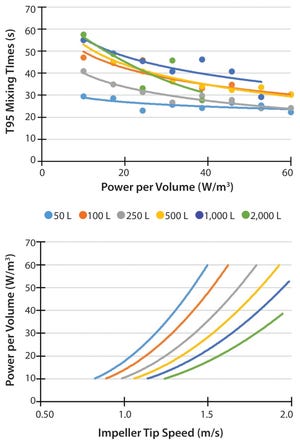
Figure 1: Highlights of requirements and features to be considered during single-use bioreactor selection and evaluation process; (A) highlight of key concepts for consideration; (B) features of the Thermo Fisher Hyperforma 2,000 L S.U.B.
There is ever increasing pressure for the biopharmaceutical industry to drive toward higher efficiency and lower costs. Compared to the past, target markets for many drugs typically are becoming smaller, and so-called blockbuster drugs are becoming more the exception than the rule. Regulatory agencies have continued to increase the pressure on drug makers to meet increasing quality standards and accept higher levels of responsibility. Furthermore, customer pricing, healthcare markets, and recent biopharmaceutical pricing scandals all add incentives toward more efficient processes.
Naturally, improving operational efficiency includes many aspects, including but not limited to drug discovery, preclinical testing, process development, clinical manufacturing, clinical trials...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.