November-December 2023 Featured Report
HTTPS://WWW.ISTOCKPHOTO.COM
It’s no secret that mammalian cell culture is the predominant production system in the biopharmaceutical industry. Microbial expression has been around longer, not least because of the early influence of the brewing industry. But once Chinese hamster ovary (CHO) and other animal cells could be cultured at scale with reliable results, that approach soon “disrupted the paradigm,” as our friends in marketing like to say. Before long, products from mammalian cell culture were dominating the development pipeline — despite the challenges in applying the technology.
Mammalian cells are fragile and finicky. Anyone who’s worked with them knows well their “high-maintenance” needs when it comes to temperature, feeding, mixing methods, and other aspects of a culture environment. The cells are also vulnerable to attack by adventitious agents, many of which can present a threat to recipients of mammalian-expressed protein drugs. But the capabilities of such cells for assembling complex prote...
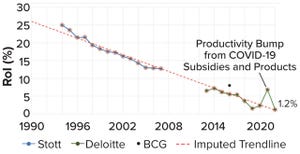
Figure 1:
The current decline in biopharmaceutical research and development (R&D), as measured by dollar returns on investment (RoI); data points are from assessments of internal rate of return (IRR) by Stott (blue,
3
), Deloitte (green,
4
), and Boston Consulting Group (BCG) (black,
5
). This figure is adapted from Stott.
The biopharmaceutical industry has entered into a creativity crisis. Despite billions in revenue, it has become a stagnant behemoth, struggling to deliver new therapies near the pace or price that the world needs. The industry’s institutional reputation is almost as low as it can get. And although “big-pharma” profits remain buoyant, research and development (R&D) productivity is declining steadily — an awkward fact for an industry that depends so greatly on innovation to maintain healthy margins (Figure 1).
At its core, our industry’s problem is cultural. We cling to a dogma eliding the important distinction between a scientific breakthrough and a new drug. To this day, we say that...
A scientist performs agroinfiltration of Nicotiana benthamiana leaves.
(
CHANDRES, HTTPS:// COMMONS.WIKIMEDIA.ORG/WIKI/FILE:AGROINFILTRATION.JPG
)
Although a 1972 United Nations (UN) resolution prohibits member states from using biological weapons on military personnel and civilians (
1, 2
), a lack of robust verification protocols has enabled several countries to develop such agents (
3
). Several groups that the UN considers to be global terrorist organizations also are establishing bioweapon capabilities (
4
). Meanwhile, potentially pandemic infectious diseases continue to pose public-health threats. Consequently, research remains active in vaccine and therapeutic development for bioterrorism mitigation and other biodefense applications.
Recombinant antibodies (rAbs) represent a relatively new and highly promising set of tools in that context. In a 2011 review, Froude et al. explain that such products could bolster biosecurity in several ways (
3
). Because rAbs have different mechanisms of action (M...
HTTPS://WWW.ISTOCK.COM
Production of therapeutic proteins typically involves culture of genetically engineered hosts, such as Chinese hamster ovary (CHO) cells. Expressed proteins then are harvested, purified, filtered, formulated, and ultimately, filled into vials to be packaged as products for administration by injection or intravenous infusion. Biopharmaceutical manufacturers and industry suppliers continue to improve the productivity of their expression systems by applying rational approaches for single-gene overexpression, knockout, or knockdown (
1
). But as I learned from Martin Fussenegger (professor of biotechnology and bioengineering in the Department of Biosystems Science and Engineering at ETH Zurich as well as professor of life sciences at the University of Basel, both in Switzerland), advances in synthetic biology now enable pharmaceutical scientists to investigate different production and treatment paradigms, including means for tunable in vivo expression of therapeutic proteins.
Much of Fu...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.