September 2023 Featured Report
STOCK.ADOBE.COM
Antibody–drug conjugates (ADCs) are straightforward as a concept. When a cytotoxic small-molecule drug is attached to an antibody raised against a specific molecular receptor, it theoretically creates a highly targeted and effective therapy. And the ability to target cells precisely has obvious applications for indications such as cancer.
However, in practice, developing and manufacturing ADCs has proven difficult, said Janice M. Reichert, chief operating officer of The Antibody Society, which is an international nonprofit organization that supports antibody-related research and development (R&D). In an email to BPI, she stated that “ADCs are complicated to develop because of the additional factors that must be considered, such as the nature of the linker and the payload and the location and the amount of the payload conjugated to the drug. As with standard antibody therapeutics, a suitable target antigen and the right patient population also need to be identified.” She added, “Clinical de...
On the cover: an artist’s rendering of antibody molecules with linker–toxin payloads attached.
HTTPS://STOCK.ADOBE.COM
Antibody-conjugate technologies have been pushed out of the limelight somewhat in recent years by mRNA/oligonucleotides and cell/gene therapies. Yet while many people were talking about the “next big thing” — and remember, the next big thing in cancer treatment not so long ago was antibody–drug conjugate (ADC) therapy — this now-maturing segment of the biopharmaceutical industry has begun proving its worth. As of November 2022, the US Food and Drug Administration (FDA) had approved 14 different ADCs for cancer indications. Seagen has led the way in commercializing four products, two on its own and two in partnership with other companies. Genentech (Roche) has commercialized two. Pfizer has two through its acquisition of Wyeth, and AstraZeneca has one of its own and one in partnership with Daiichi Sankyo. Companies with one marketed product include ImmunoGen, ADC Therapeutics, Immunomedic...
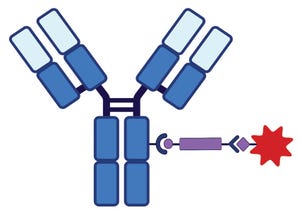
Figure 1A: Antibody–drug conjugate (ADC) structure and delivery pathways; linker chemistry determines the mechanism by which cytotoxin release occurs. This schematic shows a monoclonal antibody (MAb) conjugated to a linker (purple bar)–cytotoxin (red star) combination. Conjugation chemistry typically involves lysine or cysteine amino acids on the MAb.
The pharmaceutical industry has come a long way since 1907, when Paul Ehrlich coined the term “magic bullet” for treatments that could target disease-causing cells while sparing normal, healthy cells (
1, 2
). Researchers began investigating targeted cytotoxins as therapeutic bioconjugates in the late 1980s. Groups led by Ellen Vitetta at the University of Texas Southwestern Medical Center (
3
), Ira Pastan at the US National Institutes of Health (
4
), and John R. Murphy at Boston University (
5
) fused truncated
Pseudomonas
exotoxin (PE) and diphtheria toxin (DT) to single-chain monoclonal antibody (MAb) fragments and peptide hormones such as transformin...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.