Effect-Based ComplianceEffect-Based Compliance
Regulatory compliance is a competitive team activity that creates real value reflected in the bottom-line accounting of company profitability. Champions earn freedom to operate for their companies; losers are enjoined, have products seized, and/or are prosecuted for their (mis)deeds. The players and fans use FDA form 483s and warning letters as metrics for measuring relative standing.
The operational paradigm is that “employees” tend to define competitors in the context of a company’s market. In the regulatory game, competitors are less well-defined and can be anyone or anything that could thwart a company’s freedom to operate. Examples include regulatory authorities, new technologies, other companies (by raising performance bars), intraorganizational conflicts, and misdirected business drivers. So competitors can be people, individually and collectively, organized entities, and even ideas. Adept players recognize the complexity of the playing field: It is not level, and it coexists with a multiplicity of other playing fields on the corporate landscape.
Risk management is the new mantra. At high levels, most corporate directives today focus primarily on risk avoidance with risk defined as the possibility of loss, injury, disadvantage, or destruction. Proactive management efforts are those intended to acceptably mitigate or accommodate potential failure, which is anything that could negatively affect a company’s strategic goals.

Strategic management requires thinking about the potential effects of each task leading toward an objective rather than a simplistic addition of tasks to achieve a determined result … unless, of course, everything always works just as planned. ()
In the regulatory sector, quality assurance groups have effectively been performing quality audits to identify and realistically underwrite regulatory risk since the 1970s inception of the GxPs. Today, the US FDA embraces risk management as an appropriate tool to prioritize resources, and we find clear support of “value-add” in FDA’s Quality Systems Inspections Technique (QSIT) program. Champion organizations are given regulatory latitude afforded by trust and respect for knowing the right way to do business.
The Construct
It thus becomes imperative to create an appropriate environment for individual activities to engender organizational effectiveness. Indeed, personal responsibility is a hallmark of the Food, Drug, and Cosmetic Act (1) and reflects a core value of FDA compliance and enforcement policy. In the FDA’s eyes, individuals actively participate in unlawful conduct, allow it to happen by passively tolerating violations, or fail to take steps to learn that violations are occurring.
Our Effect-Based Compliance (EBC) concept provides a flexible and adaptable framework that any company at any development stage can implement. “EBC” actions are conceived and planned in a systems framework that considers the full range of direct, indirect, and cascading effects, which may (with different degrees of probability) be achieved by the application of different instrumentalities. The central tenet of effect-based operations is that we can somehow purposefully shape the interactions of critical players in our environment to produce both individual and overall behavior that meets organizational needs.
Most companies today use objectives-based planning models to assign tasks and identify metrics. The strategy-to-task linkage of this model relies on translating strategy at one level into objectives at the next lower level and on down until, finally, discrete, operational actions and objectives are defined that support higher-echelon objectives. This is a linear process driven by hierarchical structures (Figure 1).
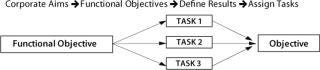
Figure 1: ()
Under the effect-based construct (Figure 2), it is assumed that each task performed has one or more causal links that ripple throughout an internal and external (competitors) system and that ultimately have either a positive or negative influence on the desired outcome. The idea of one task producing more than one effect at multiple levels is an evolutionary step forward.
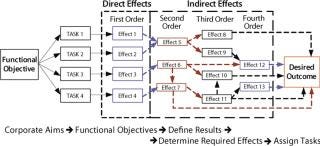
Figure 2: ()
Those causal links can be divided into two broad types of effects: direct and indirect. Direct effects are the immediate consequence of a task. They include physical effects (created through physical alteration of an object or system), functional effects (how a change directly or indirectly affects the ability of a system to function properly), collateral effects (positive or negative outcomes that result when something occurs other than intended), and psychological effects (affecting the mental domain of a population).
Higher-order indirect effects come through intermediate effects or mechanisms, producing and/or leading to a final outcome or result. These usually take time to develop and are often hard to recognize or measure. In general, the higher the order of an effect, the harder it is to predict or measure. Targeting tasks X, Y, and Z does not automatically mean a higher-order effect will be achieved.
Indirect effects include cascading effects, which ripple through a system and often influence other systems in the process. These effects usually transit across nodes that are common and critical to related systems. Although these effects may ripple upward or downward, they generally ripple downward. Cumulative effects result from aggregates of many direct and/or indirect effects. They may occur all at the same level or at different levels, typically at higher levels of employment. Last, there are indirect systemic effects that affect the operation of a given system.
Complexity
Effect-based thinking requires a unique intellectual approach because innate complexity is reflected in the basic rule set for effect-based efforts:
Actions affect anyone who can observe them (directly or indirectly).
Effects are both physical and psychological.
Effects occur simultaneously on operational, strategic, and global levels of interaction — and in multiple arenas.
All are interrelated and cumulative.
The key to effect-based thinking is a systems approach. Systems’ thinking is about “synthesis” or “synergy” (putting things together), and it is in direct contrast with analysis (taking them apart). These are complementary processes. The key to the future is synthesis. Analysis focuses on structure and reveals how components work. Synthesis focuses on function and reveals why components operate as they do. Analysis yields knowledge; synthesis yields understanding.
COMPLEMENTING OPPOSITES
Systems-based thinking reverses the three-stage order of analytical thinking, which is as follows:
1 — Decomposition of what needs to be explained
2 — Explanation of the properties of the parts taken separately
3 — Aggregating those explanations into an explanation of the whole.
The systems approach reverses them:
1 — Identify a containing whole (system) of which the component to be explained is a part.
2 — Explain the behavior or properties of that containing whole.
3 — Then explain the behavior or properties of the component in terms of its role(s) or function(s) within its containing whole.
These two approaches should not (but often do) yield contradictory or conflicting results; they are complementary.
Development of that complimentarity is a major objective of systems thinking. The performance of a system depends more on how its parts interact and interrelate than on how they act independently of on another. If each part of a system, considered separately, is made to operate as efficiently as possible, that system as a whole may not operate as effectively as possible. Trade-offs are necessary to optimize all variables.
Opportunity and Challenge
The pace of biotech innovation has exceeded the ability of process models to keep up. Analysts can trick legacy models into representing many complex phenomena, but most modeling and analysis still encourages a mechanistic view. Paradigm differences and communication problems reflect cognitive dissonance similar to what occurs across classic culture gaps.
As companies wrestle with various models to harness the capabilities of their institutional values and organizational structures, most seek to incorporate emerging technologies into innovative operational concepts for synergy.
The ultimate objective should be to unleash the human potential within. This approach is evolutionary, not revolutionary, and it delivers only short-term results in operational or strategic decisions. No one is compelled to radically modify their structures to field competitive capability.
The occurrence of unexpected results bewilders many people. In the pharmaceutical business, the surprise may be the loss of a lead compound — or it may be the brilliant success of products with but mediocre expectations. Consultants and companies analyze old reports to try to explain such outcomes, but causes are all too often ascribed to phenomena such as bad luck, serendipity, or genius. Because such analyses may apply to current scenarios and/or looming threats of counterfeit drugs and terrorism, a historical digression seems appropriate.
Years ago, concurrent with the 1982 Tylenol tampering incident in Chicago, two familiar companies dominated the North American eye-drop market, one with 60% market share and the other with 30%.
Those numbers had been constant for years, and no amount of sales effort could change them — until copycats tampered with the leader’s packages, lacing them with acid and leaving them on shelves for unsuspecting consumers. It was easier for the perpetrators to select the market leader because (in light of the Tylenol incident) the lagging company had already transferred to tamper-resistant, shrink-wrapped, and glued cartons. Meanwhile, the market leader had no tamper resistance. Overnight their market shares reversed as a consequence of unrelated actions. The reason for moving to shrink-wrap glued cartons in the first place, however, was to present a safer product. The business driver had been a belief that consumers would choose the “safe” product, but the decision-makers never expected it to be precipitated by a tragedy.
Clearly, our world is not constrained to fit a linear model; many situations are nonlinear. A linear organization is evocative of smokestack industries with assembly lines, manufacturing and delivering products within prescribed parameters. Their principles of management are used at predictable times and places for maximum effect. Today’s nonlinear environment uses dispersed activities, bringing components and elements together at the last moment and exerting simultaneously efforts at several depths to maximize efficiencies and minimize costs.
THE BUTTERFLY EFFECT
People have always wanted to be able to predict the weather. Meteorologists developed theories of air-mass interactions and seasonal patterns that gave us hope that a working model would be available as more sophisticated computers emerged. In the 1960s, a scientist named Edward Lorenz at the US Weather Bureau built an incredibly detailed program to simulate weather patterns. Using Newton’s Laws and others developed from them, he wrote equations for how weather systems develop while interacting with terrestrial and atmospheric forces. He ran his program on a computer that analyzed those equations to make predictions of future weather. The output appeared promising, but he repeated his calculations as a precaution against errors. With the same starting data, only rounded off a few decimal places to save time, the second answer was completely different from the first! Lorenz had stumbled upon a basic characteristic of complex, nonlinear equations: Their results are very sensitive to exact initial conditions. Seemingly minor changes in initial data lead to tremendous effects downstream. Discussions of this phenomenon gave rise to the famous Butterfly Effect idea, which postulates that a butterfly flapping its wings in the Brazilian rainforest today may cause a hurricane in the Caribbean a few weeks from now.
Small interactions in far-off places can cause big changes. Two recent and publicized examples involve ingredient contamination. The first was the addition of melamine to pet food in 2007, and the second is the addition of “oversulfated” chondroitin sulphate to raw heparin in 2008. The exact source of those contaminations will probably never be known because potential sources span many workshops and small providers. Companies using them never contemplated testing for such dangerous additives because they do not naturally occur in the feedstock materials — and you simply cannot test for everything. Pets and people died. It is unlikely that the perpetrators anticipated deaths that could trace back to them, but the effect has been devastating to all companies involved, with ripple effects that even negatively affected the Christmas sales of children’s toys in an entirely different industry.

Figure 1:
ALFONSODIAZ (WWW.SXC.HU)
Our organizational imperative is to abandon the simple determinism that makes us comfortable. We like to think we can reliably predict performance, and most of the time we’re right. However, we can only assign pr
obability of success or failure for a given activity. We use our own accumulated assessments (a strategy of repeated trials to see the range and distribution of possible results), or we judge by previous performance. The practical lesson is to always try injecting positive factors into starting conditions — a team commitment, social consciousness, financial rewards (stock options), a visit from the CEO — and train repeatedly under stressful conditions to explore the entire range of outcomes. The developing science of chaos and complexity provides a mathematical theory for what good leaders have been doing empirically all these years.
Chaos Theory By Any Other Name
Pharmaceuticals are critical to the social, economic, and political stability of every country. No other sector of the economy is more dependent on consumer confidence. Like food products, drugs are highly vulnerable to deliberate and/or accidental disruption, as the Japanese discovered horrifically when they experienced random mass poisoning of food and over-the-counter drugs in the 1970s. Terrorism is an unusual adversary because its perpetrators are neither perceived as competitors nor simple criminals.
The effect-based compliance movement is timely, interesting, and important — and it poses a grand challenge to the industry. In the past we created stability and understanding through a metaphor of the world as a giant, clockwork machine governed by linear relationships. Organizational foundations were based on the empirical sciences and premised on basic concepts promulgated by Sir Isaac Newton almost 300 years ago (e.g., the basic concepts of cause and effect with results being proportional to the forces applied). Common-sense observations of everyday life reinforce that paradigm.
Our goal is to “manage at the edge” between that Newtonian paradigm and the quantum paradigm enabled by effect-based thinking, and we use linearity/nonlinearity as a metaphor to understand how to accomplish it. The information age, with its almost instantaneous sharing of data, continues to revolutionize the way companies do business. Linking facts and fact-based theory across disciplines is driving a new interdisciplinary consilience. Using effects-based thinking to consider the possible effects of our actions (rather then the more simplistic attitude toward tasks required to achieve an objective) provides more robust planning with a higher probability of actually achieving our desired outcome, which is typically higher sales and increased profits.
The FDA’s endorsement of risk management as a tool moves drug manufacturers in the right direction. Expanding this tool’s usefulness, however, to bring business value beyond the minimum level of regulatory compliance is a challenge that should be welcomed by worldwide manufacturers. It can help them maintain and grow important export markets in the face of new threats from emerging and less sophisticated competitors.
Many people erroneously believe that the determiner of future success will be the application of (bio)technology, but it depends as much on organization and leadership constructs as on technological advances. Complex interactions among factors such as motivation, leadership, commitment, and endurance are not simple. No matter how many times a scenario is run during planning, its results will always be different — sometimes only in small ways and sometimes strikingly so. The difference between success and failure in some circumstances may be as small as the flap of butterfly wings in an executive suite.
REFERENCES
1.) 2004. Federal Food, Drug, and Cosmetic Act, US Food and Drug Administration, Rockville.
You May Also Like






