Development of a Turn-Key Harvest Solution for Small-Volume BioreactorsDevelopment of a Turn-Key Harvest Solution for Small-Volume Bioreactors
June 1, 2009
Over the past 10 years, disposable bioreactors have grown from a niche tool servicing small-scale projects to a common and essential component in the CGMP production of human therapeutics (1). Recent advances in filter integration, aseptic connectors, and disposable sensing allow entire cell culture processes to be performed using only single-use components. However, harvest and clarification operations remain largely dependent on centrifugation, cross-flow filtration, and depth filtration (2), which are all techniques that have not been widely adapted to single-use implementation. Their use in harvesting from disposable bioreactors can lead to process bottlenecks (3), especially when large numbers of relatively small bioreactors are in use (e.g., for clinical and research laboratories). In such cases, the advantages of ease-of-use, disposability, and turn-key processing often outweigh the need for optimized, molecule-specific processes.
Here I report the results of a three-phase, nine-month–long study that endeavored to develop a commercially viable, turn-key solution for harvesting from single-use bioreactors. The initial phase of the work involved a paper study in which available technologies were surveyed, and a primary candidate solution was selected for experimental testing. The second phase, performed at laboratory scale, screened the variety of options available within the candidate technology to develop a primary solution. The third and final phase, performed at pilot scale, generated performance data for the primary solution tested with multiple mammalian cell lines at varying levels of viability and cell density. The outcome of this work is a standard recommendation for harvesting from mammalian cell cultures using available single-use technologies.

Figure 1:
GE HEALTHCARE (WWW.GELIFESCIENCES.COM)
Down-Selection of Candidate Technologies
In the first phase of this work, candidate cell harvest technologies were ranked according to commercial availability, single-use compaitibility, scalability, and ease of use. Candidate technologies included in the down-selection process were centrifugation, cross-flow filtration, flocculation, and normal-flow filtration. Table 1 shows the results of this down-selection process.
Only commercially available technologies were considered.
Products were ranked higher if they require little or no investment in capital hardware and little or no prepreparation (e.g., flushing and cleaning). Compatibility with common preuse sterilization techniques (e.g., gamma irradiate or autoclave) also improved a product’s ranking.
Only products capable of harvesting 1-L to 50-L cultures were considered.
Technologies that are simple to operate were assigned higher rankings than those needing complex control systems or significant process development efforts.
Table 1: Down-selection of candidate technologies
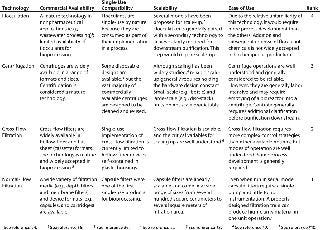
Table 1: Down-selection of candidate technologies ()
Normal-flow filtration was selected as the primary candidate technology based on its high ranking for ease of use, scalability, single-use attributes, and variety of physical formats and filtration media. Whole-cell harvest operations using normal-flow filtration are generally designed to use serially coupled filters of decreasing pore size. Most often, a depth filter or prefilter is coupled to a sterilizing-grade filter to produce material that can be immediately purified using chromatography or ultrafiltration.
For the purposes of this study, two depth materials (glass fiber and polypropylene) were considered, and two final filter options (a polyethersulfone sterilizing-grade membrane and a polyethersulfone bioburden reduction filter) were evaluated. Traditional depth filters containing cellulose or blends of cellulose and diatomaceous earth were not considered due to their inherently high extractables levels (which must be removed with a preuse flush of 50–100 L/m2 of water) and the limited availability of formats designed for single-use (11). Table 2 provides details of the filters evaluated.
Table 2: Candidate filter materials
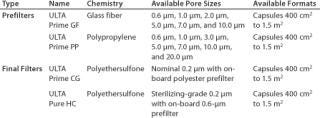
Table 2: Candidate filter materials ()
Screening
An initial experiment was performed to compare the performance of polypropylene to glass-fiber filter media. A culture of Chinese hamster ovary (CHO) cells at 4×106 cells/mL density and <20% viability (A600=2.381) was used to challenge 47-mm disc samples of 5.0-µm polypropylene (ULTA Prime PP from GE Healthcare, www.gelifesciences.com) and 5.0-µm glass-fiber (ULTA Prime GF) filters. The filters were challenged at a constant flow rate of 4 mL/min (174 LMH), and the test continued until a differential pressure of ≥9 psid was achieved. Figure 1 shows the results of the experiment.
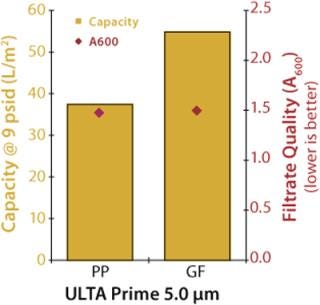
Figure 1: ()
The 5.0-µm glass fiber filter demonstrated ~46% higher capacity with a minimal difference in filtrate quality. This is not unusual given that the glass fiber media is thicker than the polypropylene media — ~550 µm and 350 µm, respectively — and thicker filtration media typically have higher particulate-holding capacities.
Upon selecting glass fiber as the primary prefilter material, two follow-up experiments were performed to select the proper pore size for mammalian cell culture applications. In the first experiment, 1-µm, 2-µm, and 5-µm disc filters were challenged under constant flow conditions (7 mL/min) using a culture of CHO-K1 cells at 2×106 cells/mL density and >90% viability. Each prefilter was coupled to an ULTA Prime CG filter disc, and the experiments continued until a differential pressure of 14.7 psid was achieved.
In the second experiment, 5-µm, 7-µm, and 10-µm disc filters were challenged under constant flow conditions (4 mL/min) using a culture of CHO cells at 4×106 cells/mL density and <20% viability (A600 = 2.381). Filtration continued until a differential pressure of ≥9 psid was achieved. No secondary filtration was performed; instead, samples were analyzed for optical clarity using an ultraviolet (UV) spectrophotometer at a wavelength of 600 nm.
Figures 2 and 3 graph the results of both experiments. As shown, the 5-µm glass-fiber filter provided the highest capacity for whole cell culture with no measurable difference in filtrate quality. Based on these results, ULTA Prime GF 5.0 was chosen as the primary clarification step for all future scale-up work. Further screening of the final filtration step was performed during the scale-up experiments.
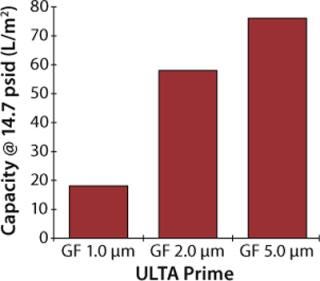
Figure 2: ()
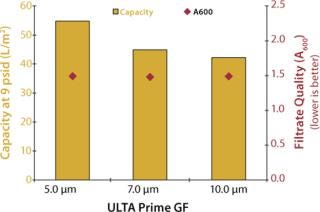
Figure 3: ()
Scale-Up and Filter Sizing
The ultimate objective of this work was to provide a set of turn-key recommendations for filter configurations capable of harvesting from various mammalian cell lines at varying cell densities and viabilities without requiring initial filter-sizing experiments. To provide such a recommendation, it was necessary to investigate the performance of ULTA Prime GF and a secondary filter (ULTA Pure HC or ULTA Prime CG) over the course of many experiments. Eight pilot-scale experiments were performed on three cell lines harvested at varying levels of viability and cell density. Figure 4 maps the characteristics of eight cultures investigated. All eight were grown in Wave bioreactors from GE Healthcare of 5-L to 10-L sizes.
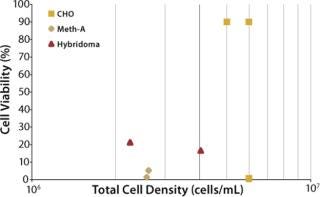
Figure 4: ()
All experiments used capsule filters under constant flow conditions. Primary (ULTA Prime GF) and secondary (ULTA Pure HC or ULTA Prime CG) filters were run in series with pressure transmitters located upstream of each. Filtrate volume and pressure data were collected as a function of time. Each experiment continued until a total pressure drop of 15 psi (1.03 bar) was observed — or until the feed vessel was exhausted. Table 3 presents experimental details for each trial.
Table 3: Experimental details

Table 3: Experimental details ()
Based on the experimental results, capacity calculations were performed for both primary and secondary filters. Sizing calculations for all filters were based on a process time of 1 hour (regardless of batch size). For primary filters, a maximum allowable pressure drop of 8 psi (0.55 bar) was chosen. For secondary filters, a maximum allowable pressure drop of 7 psi (0.48 bar) was chosen. Figure 5 presents results of the capacity calculations. The capacity of ULTA Prime CG and ULTA Pure HC (bioburden reduction and sterilizing grade, respectively) were statistically equivalent. So both options were included in the final recommendations to provide a choice between lower cost (ULTA Prime CG) and maximum quality assurance (ULTA Pure HC).
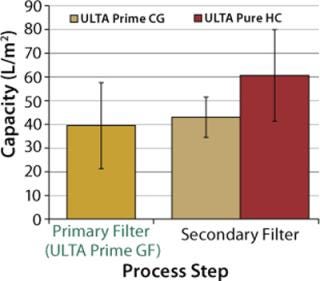
Figure 5: ()
Upon completion of the experimental work, a simple statistical analysis was performed to develop appropriate filter recommendations for varying volumes of cell culture. An attempt to produce a single solution to harvest from all culture conditions would require unreasonably large safety factors, thereby resulting in process waste for most applications. So the project goal was refined to produce a solution that could be successfully applied in 80% of all mammalian cell culture applications. A Student’s T-distribution was used to calculate that 89.6% of one standard deviation would provide a sufficient safety margin to meet that goal, and required filter areas for various batch sizes were calculated.
Table 4 shows the analytical results. Those requirements were matched to available capsule sizes for each filter grade to generate final-product recommendations. Because filters come in discrete area increments, it is impossible to generate a perfect match. To minimize process waste, a filter area tolerance of up to –8% was allowed. Table 5 presents the final recommendations.
Table 4: Filter area requirements
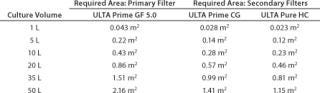
Table 4: Filter area requirements ()
Table 5: Final harvest recommendations
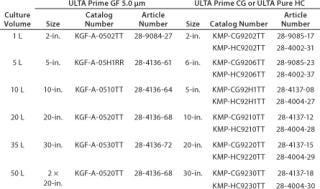
Table 5: Final harvest recommendations ()
A Suitable Platform
Although large-scale GMP manufacturing operations may require extensive process optimization, many smaller-scale operations demand turn-key solutions that enable quick turnaround and high levels of productivity. For harvest operations, glass-fiber filters coupled to a retentive membrane provide a rapid solution that can be implemented with very little preparation. Glass-fiber and polyethersulfone membranes are inherently low in extractables and have been validated for low levels of endotoxin and other contaminants. They are steam sterilizable and compatible with gamma irradiation, enabling preuse sterilization with either method.
The solution presented here provides a reliable solution designed to work in the majority of mammalian cell culture applications with no upfront process development efforts. Future efforts are planned to extend this methodology to other cell types (e.g., insect cells and yeast) and to address larger-volume reactors with a complementary technique.
REFERENCES
1.) Glaser, V 2005. Disposable Bioreactors Become Standard Fare. Gen. Eng. News 25:80-81.
2.) Shukla, A, and JR Kandula. 2008. Harvest and Recovery of Monoclonal Antibodies from Large-Scale Mammalian Cell Culture. BioPharm Int. 21:34-45.
3.) Pattnaik, PCell Culture Clarification By Depth Filtration Biospectrum Asia.
4.) Tchobanoglous, G, and HD Stensel. 2003.Wastewater Engineering: Treatment and Reusefourth edition, McGraw-Hill, New York.
5.) Ducoste, AJ. 2002. Two-Scale PBM for Modeling Turbulent Flocculation in Water Treatment Processes. Chem. Eng. Sci. 57:2157-2168.
6.) Bracco, C. Centritech® System in Cell Harvesting http://celld.com/documents-adobe/centritech-recolte-cellulaire.pdf (accessed November 2008)..
7.) Romero, J. 2007.Disc Stack Centrifugation 2L to 20,000L Scale-Up Evaluation for Mammalian Cell Clarification: Optimization of Bioreactor Harvest Conditions for Combined Cell Removal and MAb Purification Capabilities, American Institute of Chemical Engineers Annual Meeting, Salt Lake City.
8.) Van Reis, R, and A Zydney. 2001. Membrane Separations in Biotechnology. Curr. Opin. Biotechnol. 12:208-211.
9.) Van Reis, R. 1991. Industrial Scale Harvest of Proteins from Mammalian Cell Culture By Tangential Flow Filtration. Biotechnol. Bioeng. 38:413-422.
10.) Yavorsky, D. 2003. The Clarification of Bioreactor Cell Cultures for Biopharmaceuticals. Pharmaceut. Technol. 27:62-76.
11.) 2003.Application Guide AN1100EN00Using Millistak+ HC Filters for Mammalian Cell Culture Clarification, Millipore Corporation, Billerica.
You May Also Like






