Development and Qualification of a Scalable, Disposable Bioreactor for GMP-Compliant Cell CultureDevelopment and Qualification of a Scalable, Disposable Bioreactor for GMP-Compliant Cell Culture

Photo 1: BIOSTAT STR 2000 bioreactor
During the development of single-use, stirred-tank bioreactors (e.g., BIOSTAT STR bioreactors), different phases can be distinguished (Figure 1). First, a clear definition of the intended application and all related requirements should be captured in a user requirement specification (URS). Based on that, the single-use bioreactor design phase and the material selection phase are initiated, both closely linked to each other. During the proof-of-concept phase, relevant component- and product-based tests are established and realized to ensure URS compliance. Finally, the qualification can be divided into product and process qualification, in which the latter includes qualification of the production equipment procedures and parameters. This process, together with stringent change-control procedures, will positively influence batch-to-batch consistency and security of supply.
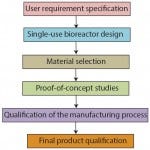
Figure 1:Phases during product
development of a single-use bioreactor
Final product qualification should include qualifying the product’s individual components and the final product using mechanical and biological tests emulating the intended application or a combination of both. Those tests should be performed on bioreactor bags derived from regular manufacturing.
User Requirement Specification
At the start of BIOSTAT STR bioreactor development, key user requirements are defined covering basic requirements of cell growth and productivity, robustness, reliability, and security of supply. Leachables from plastic materials should be considered to ensure a reproducible biological performance because such substances can affect cell growth (1). Other key requirements are proper mixing and oxygen transfer (2). The stirrer design should allow for superior mixing while minimizing shear forces.
Stainless steel processes typically include highly automated cleaning and sterilization. Single-use bioreactor set-up and change-over require a higher level of manual handling. Therefore, bag handling must be considered during the development process to make it easy-to-use and limit risks for misuse leading to loss of bag integrity.
Single-Use Bioreactor Design
Because the characteristics of stirred stainless steel bioreactors are well understood, this culture vessel design has been the gold standard for bioprocessing for decades. Based on that, the BIOSTAT STR single-use bioreactor family (Photo 1) was developed and designed from 12.5 L to 2,000 L (2). A detailed description of the single-use bioreactor design is given by De Wilde et al. (2).
The BIOSTAT STR bioreactor is equipped with fluorescence-based single-use sensors for pO2 and pH measurement and control. Sensor patches are installed and sterilized together with the cultivation bag. Modern single-use sensor technology ensures the same level of control as traditional sensors but avoids risky insertions of classical probes (3). The functionality of the probes is tested successfully by Reglin et al. (4) in a high–cell-density, 1,000 L STR fed-batch CHO cultivation using chemically defined media. Reusable probes can be inserted as an alternative if desired.
Material Selection
A single-use bioreactor bag mainly consists of various components made of polymer resins. Raw material selection for the individual components is guided by regulatory considerations, mechanical properties needed for the bioreactor’s intended use, and the need for a biological performance comparable with glass and stainless steel bioreactors (5). We followed guidelines that are applicable to polymeric materials that come into contact with final pharmaceutical products (e.g., requirements established for pharmaceutical packaging). Guidelines related to the food and medical-devices industries also can facilitate assessment of the suitability of a material for bioprocess applications (5>). Table 1 presents examples of relevant regulations that should be taken into account when qualifying raw materials of polymeric nature for single-use bioreactors.

Table 1: Examples of relevant guidelines
The Flexsafe STR bag contains internal moving components (e.g., the agitation system) that are subject to substantial mechanical forces during bag manufacturing, transport, installation, and use. Therefore mechanical properties of the raw material (e.g., hardness and tensile modulus) must be carefully considered, not only with regard to component performance (e.g., tensile strength), but also for component manufacturing (e.g., melt flow index). Once the components are fabricated out of a selected raw material, they have to be evaluated, typically in the final assembly during the proof-of-concept phase.
Proof-of-Concept Studies and Production Process Qualification
A key element in biopharmaceutical development is transfer of a cultivation process from laboratory to production scale while ensuring comparable process characteristics (6). Although a design-space approach can support successful process transfer (7, 8), scale-up remains a challenge and is “as much an art as a science” (9). Therefore, extensive process understanding and knowledge of the bioreactors and equipment in use is a prerequisite (10).

Photo 2: Tensile and flex durability tests (photos left and middle from Reference 19)
During the proof-of-concept phase, the design space with regard to agitation and aeration rate was determined, and a system characterization was conducted, in
which typical scale-up parameters were evaluated (11). De Wilde et al. describe a detailed characterization approach (2). And Reglin et al. (4) describe a successful transfer of a high–cell-density CHO fed-batch process to a Flexsafe STR 1000 bioreactor finalizing the proof-of-concept phase.
After a successful proof of concept, the final design was transferred into production, including operator training and qualification of the production equipment and procedures. Production parameters were monitored, and requalification of them was performed in regular intervals. A stringent change-control process ensures consistent quality, performance, and reliability.
As part of ensuring a high level of supply assurance, current regulatory guidelines and standards for quality systems such as ISO 9001 (12) were followed. These guidelines instruct manufactures of single-use bioreactors to control components and services obtained from subsuppliers. During supplier qualification, their quality system, their experience in offering materials for pharmaceutical or medical applications, and the suppliers reliability are critically reviewed. Suppliers of raw materials for components in direct contact with process fluids should apply a quality system meeting international standards such as ISO 9001, ISO 13485, or CFR 21 Part 820 (5). Finally, specific design parameters (e.g., critical dimensions and component delivery times) are established for all critical parts.
Final Qualification Approach
To perform a proper qualification, it is not sufficient to focus only on the qualification of the components and the manufacturing process. Qualification of the final product under relevant conditions for the intended use is absolutely critical.
By contrast with pharmaceutical plastic packaging, no regulatory guidelines have been specifically developed for the qualification of single-use bioreactors. Therefore, the qualification approach of STR bags is based on general good manufacturing practice (GMP) principles and relevant parts of existing guidelines associated with other products. For the component-based qualification approach, test procedures for individual components are implemented. The qualification approach for the entire final product is based on the verification of the materials and assemblies during the performance qualification at the application level. Therefore, a combined approach of qualifying individual components as well as qualification at application level of the final product is considered the most meaningful and effective way to qualify such a complex system (2).
Selecting and Qualifying the Proper Film Properties
Films used in bioprocess applications commonly possess a multilayer structure. Currently marketed films include a backbone and a contact layer. The backbone provides the barrier structure and consists of one or more layers, which determine the overall mechanical behavior and barrier properties. The contact layer is the surface that comes into contact with culture broth. It must be inert and have good sealing characteristics (5).
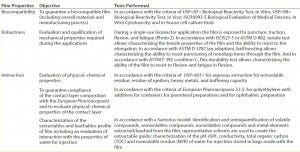
Table 2: Example approach to qualify and characterize a film for bioreactor applications
Examples for properties typically tested in accordance with regulations applicable to biopharmaceutical packaging are shown in Table 2. Some of those tests are defined to qualify the film with pass–fail criteria. Others are performed to characterize the film, without specifications.
The influence of substances leached from the film on the growth and productivity of mammalian cells has been identified as one of the major concerns related to the use of single-use bioreactors. Fenge et al. (1) summarize the approach to investigate biocompatibility of the new S80 polyethylene film used in the Flexsafe product range.
A design space (process window) for critical process parameters of film extrusion was defined to
guarantee extrusion process reproducibility within established parameters settings of the design space
guarantee film quality and reproducibility
guarantee that film properties reach the acceptance criteria when films are produced within the design space.
The properties of the film were also verified after aging it three years to establish the film’s shelf life. Both accelerated aging regulated by ASTM F 1980 (13) and natural aging conditions were considered to qualify the three-year shelf life of the film.
Qualification of Assemblies
The various connections between individual components of the single-use bioreactor were qualified to demonstrate physical and mechanical integrity of the final bag assembly. To demonstrate reliability, reproducibility, and robustness, seal strength and integrity were qualified. Robustness was qualified both on final-product level and on component level.
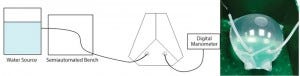
Figure 2: Water burst test with a filled bag chamber
A water-burst test (Figure 2) was developed to evaluate the robustness of critical welds. A certain nominal volume could be reached before bursting — caused by a breakage of the film rather than at the welds — of the model S80 bag (14). Comparing these results with those obtained from other commercially available films (not shown) confirms superior robustness of the new S80 polyethylene film used for the manufacturing of Flexsafe bags. For the conversion of the STR bag family to the new S80 film, each weld of the single-use bioreactor chamber was subject to tensile strengths, color invasion, and water-burst testing. The color invasion test allows identification of the presence of microchannels in a weld. Different film lots were used to conduct those tests.
Successful Realization of the Final Bioreactor Product
Ensuring State-of-the-Art Bioreactor Bag Robustness: Qualification of the STR bags with S80 film was based on a worst-case rationale relevant to the final application. Because bigger bags are exposed to larger mechanical forces, they can be considered as a worst case comparison for smaller sizes. Three film lots were used for the final qualification approach, and a representative bag quantity was tested. Additional robustness trials will be performed routinely on bags from regular production to confirm the results of the initial qualification.
The parameters of the robustness trial were chosen based on the typical characteristics of a mammalian cell-culture process. In the 21-day trial, a maximum filling volume, maximum stirrer speed, 40°C, and 30 mbar overpressure were applied. Additionally, an aeration test and a pressure stress test were conducted following the 21-day period.
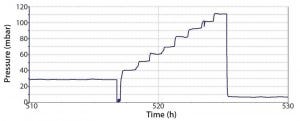
Figure 3: Pressure plot obtained during a robustness trial of a Flexsafe STR 1000, including final
aeration and pressure stress test
During robustness qualification and routine testing, a pressure of up to 50 mbar was evaluated. Additionally, a worst-case pressure stress test on a selected number of single-use bioreactor bags was performed. That consisted of increasing the pressure from initially 30 mbar to 110 mbar in 10-mbar steps (Figure 3). Every pressure-step increase was maintained constant for one hour. Before and after the robustness trial, a visual inspection of the whole bag, seams, and the agitation system was performed. Table 3 lists the defined pass criteria for the robustness trials.
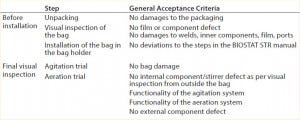
Table 3: Assessment of the robustness trial
Sterilization Validation to Ensure Risk-Free Cell Cultivation: The Flexsafe STR system is sterilized by gamma irradiation. The first step in the validation of a gamma sterilization cycle with a security assurance level (SAL) of 10–6 described by the ISO 11137 (15) is assessment of the final bag product’s bioburden load.
Determination of the bioburden of the Flexsafe STR family is based on an in-house method in accordance to ISO 11737 (Sterilization of Medical Devices: Microbiological Methods, Part 1 — Determination of a Population of Microorganisms on Products) (16).
A dose-mapping study of the Flexsafe STR product family was performed according to ISO regulations (15) and based on a worst-case rational. Because it is directly linked to product density, including its packaging, we defined the packaging and performed a transportation test (described below) before dose mapping. The verification dose is calculated to achieve SAL 10–1 according to ISO 11137 (15). Individual sterility testing was performed on a specific number of STR bags.
Packaging Validation to Ensure Full Robustness in a World without Boundaries: Packaging was qualified by a transportation test simulation in accordance to ASTM D 4169-DC 2 (17) to assure robustness and protection against mechanical handling (impacts and drops), vehicle vibration (truck and aircraft cargo), and stacking. The Flexsafe STR 1,000-L unit was tested and was taken as the representative for the STR 500-L unit because of their nearly identical packaging dimensions. The Flexsafe STR 50-L and 200-L models were tested because they differ in terms of unit per pallet and height. Furthermore, the Flexsafe STR 2000-L unit was successfully tested as a worst-case configuration in terms of height and weight.

Table 4: Stability of water for injection in accordance with the European and US pharmacopoeias
Stability of Water for Injection: Apart from the mentioned qualification approaches for robustness and sterility, leachable data are provided. The stability for water for injection is specified by the monographs of the US and European pharmacopoeias. Tests were applied to gamma-irradiated Flexsafe STR bags (Table 4) and documented in a validation guide. Results from the stability of water for injection are not considered as release criteria but serve as comparable data for the pharmaceutical industry because mammalian cell-culture media are used during the application.
For the stability of water-for-injection trials, three subfamilies are defined, considering the construction of the agitation system. Three bags per subfamily from three different film lots were tested. The conditions were 40 °C for 21 days with identical working volume, pressure, and agitation speed as for the robustness trial, comparable to a worst-case cell-culture application. In accordance with US Pharmacopeia <85>, endotoxin values were determined.
Extraction with Water and Ethanol: Extractables can directly influence the quality of a final product. Therefore, in each pharmaceutical production step, potential contamination with extractables has to be assessed. Detailed knowledge of the chemical nature of extractables is essential even for trace amounts.
The analytical scheme was developed in line with the concept that a bag is made up of a large number of different polymeric materials. Potential extractables could consist of respective breakdown products, residual monomers, and oligomers from manufacturing residues and additives of the different polymers. Such a broad spectrum of substances represents a complex analytical challenge that can be managed only by combining different analytical methods. Typically, various mass spectrometry methods are used and extractables are investigated, with the amount and nature determined.
For developing the analytical scheme, the two most commonly used solvents (reverse osmosis water and absolute ethanol) were chosen to create a database covering all extractables. For this study, a rational approach was used that considered the different components of the Flexsafe STR family and a worst-case ratio of film surface to volume. The extraction conditions (40 °C for 21 days) are considered to be comparable to a typical mammalian cell-culture process.
Long-Term Flexsafe Bag Stability: Shelf-life qualification of the Flexsafe STR bags is comparable to the verification of STR bags made of S40 film (18). Based on a risk assessment, the STR 2,000-L, 1,000-L, and 200-L models were chosen to determine robustness, stability of water for injection, and functionality of the optical sensors after accelerated ageing in accordance to ASTM F 1980 (13) and natural ageing after two years. Storage conditions for accelerated aging are 225 days at 40 °C and 75% humidity. This correlates to two years natural aging.
Conclusions
Unlike other single-use bioreactor designs available on the market, the BIOSTAT STR bioreactor family is unique in its design and performance because its design is similar to that of conventional stainless steel stirred tank bioreactors. For development and qualification, specific tests were selected or developed to ensure full robustness, reliability, and lot-to-lot consistency. Robustness, cell culture performance, and quality of this disposable bioreactor family are based primarily on the selection of suitable polymer materials and a well-defined and controlled production process to ensure that critical quality attributes are consistently met. Furthermore, an intelligent, application-driven qualification approach both on component level as well as on final product level was established that emulates the intended application of the advanced single-use bioreactor platform. This ensures a holistic qualification approach starting from the raw materials to the final single-use bioprocessing bag product in its intended application.
Full functionality and consistent and reproducible performance could be confirmed, and biological compatibility was proven by mammalian cell-culture trials (1, 4). Superior and consistent robustness was confirmed in worst-case application trials and pressure tests. All relevant and recommended tests according to US and European pharmacopoeial monographs and an extractable study were conducted and passed successfully. Finally, qualification of the BIOSTAT STR single-use bioreactor family confirmed that the user-requirement specification (which was defined at the starting point of the product development) was consistently met.
The risk-based approach of testing at component level and on final-product level, in the intended application, ensured the successful development of a robust single-use bioreactor. That bioreactor is not only highly comparable with existing stainless-steel bioreactors, but also meets industry requirements with regard to quality, robustness, and assurance of supply.
Acknowledgment
Thanks to the R&D team in Aubagne and Göttingen.
References
1 Christel F, et al. Consistently Superior Cell Growth Achieved with New Polyethylene Film Formulation. BioProcess Int. 12(8) 2014: S34–S37.
2 De Wilde D, et al. Enhanced Scalability in Single-Use Bioreactors. BioProcess Int. 12(8) 2014: S14–S19.
3 Noack U, et al. Single-Use Stirred Tank Reactor BIOSTAT® CultiBag STR: Characterization and Applications. Single-Use Technology in Biopharmaceutical Manufacture. Eibl R, Eibl D, Eds. John Wiley & Sons, Inc: New York, NY, 2011; 226–2404.
4 Reglin R, et al. Validation New Flexsafe Film in a CHO Fed Batch MAb Process in the BIOSTAT STR 1000L. BioProcess Int. 12(8) 2014: S53–S57.
5 Barbaroux M, Gerighausen S, Hackel H. An Approach to Quality and Security of Supply for Single-Use Bioreactors. Adv. Biochem. Eng. Biotechnol. 138, 2014: 239–272.
6 Vorlop J, Lehmann J. Scale-Up of Bioreactors for Fermentation of Mammalian Cell Cultures, with Special Reference to Oxygen Supply and Microcarrier Mixing. Chem. Eng. Technol. 11(1) 1988: 171–178.
7 Xing Z, et al. Scale-Up Analysis for a CHO Cell Culture Process in Large-Scale Bioreactors. Biotechnol Bioeng. 103(4) 2009: 733–746.
8 Varley J, Birch J. Reactor Design for Large-Scale Suspension Animal Cell Culture. Cytotechnol. 29(3) 1999: 177–205.
9 Ma N, Chalmers JJ, Mollet M. Aeration, Mixing, and Hydrodynamics in Bioreactors. Ozturk SS, Hu WS, Ed. Cell Culture Technology for Pharmaceutical and Cell-Based Therapies. CRC Press, New York, NY, 2006; 225–248.
10 Catapano G, et al. Bioreactor Design and Scale-Up. Cell and Tissue Reaction Engineering. Eibl R, et al., Eds. Springer, Berlin-Heidelberg: Berlin, Germany, 2009; 173–262.
11 Löffelholz C, et al. Dynamic Single- Use Bioreactors Used in Modern Liter and m³ Scale Biotechnological Processes: Engineering Characteristics and Scaling-Up. Adv. Biochem. Eng. Biotechnol. 138, 2014: 1–44.
12 ISO 9001:2008 Quality Management Systems — Requirements. International Organization for Standardization: Geneva, Switzerland, 2008.
13 ASTM F1980:2007 Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices. American Society for Testing and Materials: West Conshohocken, PA, 2007.
14 Vachette E, et al. Robust and Convenient Single-Use Processing with Superior Strength and Flexibility of Flexsave Bags. BioProcess Int. 12(8) 2014: S38–S42.
15 ISO 11137:2006 Sterilization of Health Care Products. International Organization for Standardization: Geneva, Switzerland, 2006.
16 ISO 11737-1:2006 Sterilization of Medical Devices: Microbial Methods, Part 1 — Determination of a Population of Microorganism on Products. International Organization for Standardization: Geneva, Switzerland, 2006.
17 ASTM D4169:2009 Standard Practice for Performance Testing of Shipping Containers and Systems. American Society for Testing and Materials: West Conshohocken, PA, 2009.
18 Weber A, et al. Development and Qualification of a Scalable, Disposable Bioreactor for GMP-Compliant Cell Culture BioProcess Int. 11(4) 2013: S6–S17.
19 Teniers C. How Laminates with EVAL™ EVOH Film Improve the Performance of VIPS; http://oisd.brookes.ac. uk/ivisnet/resources/papers/3A_Cynthia Teniers – revised copy.pdf.
Anne Weber is a scientist, upstream technology, R&D; corresponding author Davy De Wilde is director of marketing, fermentation technologies ([email protected]); Sébastien Chaussin is R&D program leader, fluid management technology; Thorsten Adams is product manager, fermentation technologies; Susanne Gerighausen is director of quality; Gerhard Greller is R&D director, upstream technology; and Christel Fenge is vice president of marketing, fermentation technologies at Sartorius Stedim Biotech.
You May Also Like






