Disposable pH SensorsDisposable pH Sensors
This paper describes the design, development and validation procedure for a novel, single-use gamma-stable electrochemical pH probe jointly developed by Sartorius Stedim Biotech SA and Metroglas. This new, single-use pH sensor offers a range of pH measurements (from 0 to 11 with ±0.1 precision) and features a one-point calibration process in its storage solution that provides a fast and easy pre- and post-use sensor performance check. Also described is a specific encapsulation device designed to integrate the pH probe into single-use containers used to hold, mix, store, transfer and transport biopharmaceutical fluids and drug products. Finally, a case study is detailed that outlines the potential application of a combined single-use pH probe and mixer technologies for buffer formulation applications.
Single-use technologies have been widely adopted by the biopharmaceutical industry for the management of buffers, media, cell culture, harvest, cleaning agents and intermediate products. As single-use technologies are migrating towards more critical and complex process steps, they must now be combined with single-use process control and monitoring devices and their associated instruments. The capability for single-use bag suppliers and end-users to apply critical process controls, such as pH, temperature and conductivity, is instrumental to the extension of future single-use products into more critical biomanufacturing operations. The new single-use pH sensor described in this paper provides a leap forward in the areas of single-use mixers and bioreactors, and could help our industry to move a step closer to the reality of the “totally disposable factory” with more integrated technologies and control systems.

Figure 1:
Flexel® 3D LevMix System for Palletank® with Single-Use pH Sensor
Market Requirements
The primary target applications for the integrated pH sensor system include buffer and media preparation, as well as viral inactivation. Particularly in viral inactivation, the precise control and documentation of actual pH values can be essential for the optimization and certification of the process. If one looks at the generic process steps in the manufacturing of vaccines or monoclonal antibodies, one can quickly identify where advanced single-use systems are required. These systems have to include fully integrated single-use probes to improve the level of monitoring and control. Obviously, all mixing applications along the process chain play a major role, as mixing efficiency and reliability are key to ensuring consistent product formulation and quality attributes. Liquid-to-liquid or powder-to-liquid applications such as buffer preparation, media preparation, viral inactivation, the homogenization of purification intermediates and/or multiple suspension and resuspension steps comprise the backbone of upstream and downstream operations. Among these, final formulation before form and fill is presumably one of the most critical mixing operations and thus requires accurate monitoring and management of the mixing performance. All of the mixing applications previously mentioned have one key criterion in common: very often, the pH value is used as the key indicator to qualify mixing effectiveness and efficiency.
In fact, monitoring the pH value alone simply provides information about the actual mixing status. Hence, the adjustment function of such a system by the automated addition of acid or base fluids becomes even more important. Probes must be calibrated before and after use. Gamma irradiation resistance, as well as the subsequent calibration, have turned out to be major hurdles for all the single-use probes provided by numerous manufactures to the industry so far. These challenges, combined with the need to meet the stringent regulatory requirements, have prompted the suppliers of single-use systems to find new and innovative approaches to overcome the obstacles encountered in the past.
Basic Principles of pH Measurement
The pH scale has been defined as the negative logarithm of hydrogen ion activity (1). This quantity is not directly accessible and has to be determined by referencing it to other ion activities. The measurement of electrochemical potentials has traditionally been the method of choice, as electrochemical potentials are referenced to the standard hydrogen electrode with great accuracy by national standard institutions. The quantitative relationship of the electrochemical potential is given by the Nernst equation. For hydrogen ions it takes the linear form:
E = E0 − RT/F ln (a H+) where E is the measured potential, E0 is the zero potential, R is the Boltzman Constant, F is the Faraday Constant and aH+ is the hydrogen ion activity.
The measured potential, E, depends on E0, which needs to be defined. A pH glass electrode consists of two electrochemical cells — an indicator electrode and a reference electrode — combined in one unit. By convention, the design is adapted such that the electrode potential reads 0 mV at pH 7 and 25 °C (DIN 19263:2007-05). To read pH values accurately, pH electrodes are calibrated by using pH standard solutions; that is, the slope and the electrode zero point are determined. The pH electrodes follow the theoretical Nernst equation slopes within a small margin of error, whereas the zero point may need to be adjusted over a wider range of up to ±15 mV. For complete measurement confidence, there are standard solutions that are fully traceable to primary standard materials. It is also important to note the uncertainties associated with the standard buffers used for calibration, as these will put a limit on the maximum achievable measurement accuracy. For more information on pH measurement accuracy, see DIN 19268:2007-05 (D). Unlike other pH sensors such as optical devices, pH glass electrodes relate directly to the electrochemical definition of the pH scale (2). It is therefore highly desirable to have the trusted pH glass electrodes available for single-use bioprocessing equipment. pH glass electrodes also exhibit one of the widest dynamic ranges available in any type of sensor, a linear response over almost 14 orders of magnitude.
Single-Use pH Probe Design
The first challenge to overcome when integrating a single-use pH probe into bags concerns the single-use assembly production and gamma sterilization processes. The electrode has to be inserted into the bag; it must withstand gamma sterilization and then be stored in a sterile liquid environment until the time of use. The second challenge is to enable a performance test prior to use and allow calibration of the sensor by the end-user — without risking the sterility of the single-use assembly — before inserting the sensor into the bag in a non-invasive way. The design of the single-use pH sensor was adapted to meet these stringent requirements. A short body facilitates the packaging and handling of the sensor. Specific gels were chosen for both the internal and external reference electrolytes, which allow the probe to be mounted and be ready for measurement in any orientation. The specifications are listed in Table 1.
Table 1: Specifications for the single-use electrode.
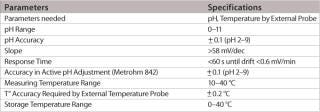
Table 1: Specifications for the single-use electrode. ()
The pH range of the modified design is as wide as with standard pH electrodes and, depending on the application and calibration method used, an accuracy level of at least 0.1 pH units or better can easily be achieved. The most important feature of this new pH probe is the ability to maintain its production specifications after the gamma sterilization process used for single-use bags. Thorough validation testing was done to assess the effect of worst-case gamma sterilization doses on the calibration buffer and the accuracy of the single-use sensor. Test results summarized in Table 2 show that the slope of the special electrodes remains virtually unchanged after gamma sterilization. However, an increase in the zero point potential is regularly observed.
Table 2: Slope and zero point values before/after gamma sterilization for 10 electrodes. Slope values are given as relative to the theoretical Nernst slope of –59.2 mV/decade (25°C).
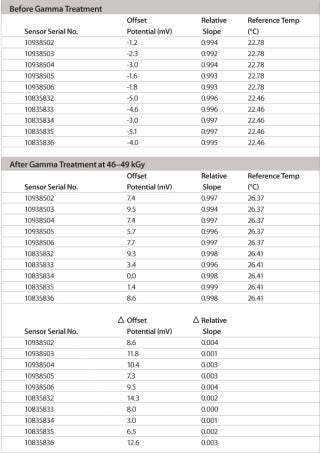
Table 2: Slope and zero point values before/after gamma sterilization for 10 electrodes. Slope values are given as relative to the theoretical Nernst slope of –59.2 mV/decade (25°C). ()
Insertion Device Design and Operations
The single-use pH sensor must be kept in a wet environment during storage, before and after gamma radiation. Also, some critical applications will require a pre-use check and calibration of the pH sensor. This calibration needs to be performed under aseptic conditions without compromising the sterility of the single-use assembly. These two critical requirements led to the development of an encapsulation device that maintains the pH probe in its calibration buffer. The patented encapsulation device is also designed to enable the insertion of the single-use pH sensor into Flexel® 3D bags without affecting sterility. The encapsulation and insertion device is composed of three essential components: the clip, the port and the silicone-moulded body and plug. The clip and the port are made of polypropylene and polyethylene plastic materials and manufactured under Class ISO 7 cleanroom conditions. The silicone-moulded part is also manufactured under ISO 7 cleanroom conditions and sealed around the pH sensor. This component ensures centring and tightness during storage in wet conditions and the pre-use calibration process, and facilitates sensor insertion (Figure 1). All the materials selected to manufacture the insertion device meet the requirements of the USP Class VI, are free of animal derivatives and are compatible with gamma sterilization at a maximum dose of 50 kGy. The storage solution is added to the closed compartment formed around the pH sensor. The Flexel® 3D bag with the sensor is gamma sterilized (25/45 kGy) and stored before use.
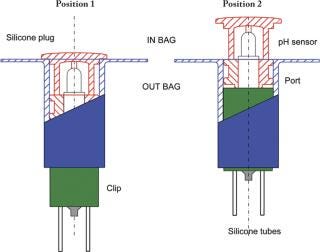
Figure 1: ()
The single-use pH sensor can be connected to a pH amplifier at any time during storage to check its potential via a patent-pending procedure. An estimate of the electrode zero point can be derived from the recorded potential because the sensor is stored in a calibration buffer whose pH is not affected by the gamma sterilization process. Effectively, the procedure constitutes a quick one-point calibration that is typically done within 30 s. Together with the previously determined slope, the two values form a set of provisional calibration values. In the validation steps that have been performed, the accuracy and precision of this procedure have remained well within the required margin of 0.1 pH units.
A two-point calibration or a multipoint calibration process is also available. It requires the flushing of the storage and calibration buffer using the set of silicone tubes and sterile filters that are part of the assembly. The first calibration buffer can be drained using a syringe connected to the sterile filter. Another rinse using a second syringe is done before the injection of the second calibration buffer. In both cases, the device needs final rinsing with Water-for-Injection (WFI). Calibration and flushing volumes are in the range of 5–10 mL and the operations are performed under sterile conditions using preconnected sterile filters. After calibration, flushing and draining, the pH sensor is ready to be inserted into the bag (Figure 2).
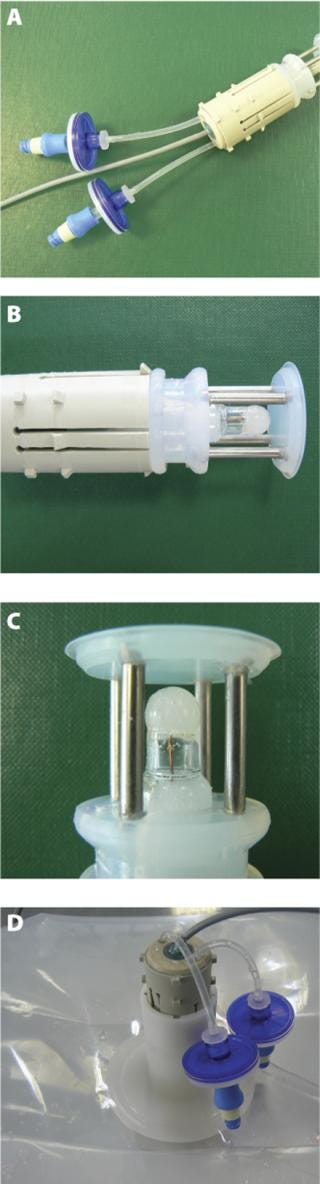
Figure 2: ()
Case Study: Buffer Mixing
In research and development environments, the quick preparation of different buffers at specific pH values is crucial. The system presented in this paper is ideally suited for active pH adjustments when the currently measured pH value is fed into a control loop that steers the dispensing of acid or base solutions into the bag. Metrohm titrators have very robust control algorithms and are well suited to adjusting the pH of a large solution volume. When approaching the target pH value, the dispensing process is slowed down in a controlled way, leaving enough mixing time to ensure that the entire volume has a uniform pH. Even when using volumes of 100– 500 L, the process can be completed in a remarkably short time. Usually, buffer substances can be precisely weighed and added to yield a certain pH. However, fine adjustments are very often needed to ensure uniformity across several batches (Figures 3 and 4).
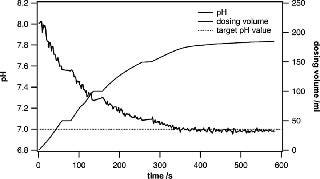
Figure 3: ()
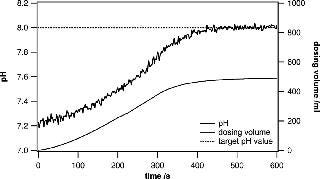
Figure 4: ()
Conclusion
The use of a gamma-stable electrochemical pH probe in single-use bioreactors, mixers and storage bags is a first step towards the future implementation of controlled single-use unit operations. Integrating a pH sensor into mixing bags allows the controlled formulation of buffers and cell culture media preparations, and can help to monitor cell cultures, chromatography, crossflow and viral inactivation processes. Further technology developments will be required to enhance the monitoring and control of other process parameters, such as pressure, temperature, flowrate and conductivity.
The development of single-use sensors in conjunction with advanced instruments, hardware and software is a prerequisite towards the implementation of future automated single-use processes.
REFERENCES
1.) Galster, H 1991.pH Measurement: Fundamentals, Methods, Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.) Buck, RP. 2002.. Pure Appl. Chem. 74:2169-2200.
You May Also Like






