Pressure Decay Method for Postinstallation Single-Use Bioreactor Bag TestingPressure Decay Method for Postinstallation Single-Use Bioreactor Bag Testing
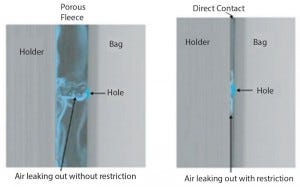
Figure 1: Porous fleece prevents masking effect
Single-use technology is well accepted today, and manufacturers’ quality assurance programs ensure leak-free single-use bags upon delivery. But what about risks involved with installation and other handling errors? Operator training and implementation of suitable standard operating procedures (SOPs) are mandatory, but should they be the only ways to mitigate the risk of failures? In addition, more companies are advocating the use of ballroom concepts (1) for the manufacture of biopharmaceutical drug substances and drug products. However, how do you prove that applied unit operations are closed and intermediates are not subjected to risks of cross-contamination?
The high value of growth media and the duration of typical cell culture processes call for the highest degree of scrutiny when setting up such runs. A leaking bioreactor would generate a significant financial loss and jeopardize a carefully timed production schedule in a good manufacturing practice (GMP) production facility. In vaccine processes, it might also pose a risk to operator safety as well as the general environment and therefore must be mitigated under all circumstances.
Postinstallation Preuse Testing Helps Mitigate Risks
In terms of regulatory framework and feasibility, there is a clear difference between integrity testing sterilizing-grade filters and testing single-use bags. According to current European GMP regulations (2) — which are the most stringent among international guidelines — sterilizing-grade filters should be tested after sterilization both before use and immediately after use. Single-use bags have no such regulatory requirement. Nonetheless, a postinstallation preuse test of the entire single-use bioreactor system (including tubing) — capable of detecting typical leaks that might have been introduced by operator-handling errors — would greatly improve risk-mitigation capabilities in single-use production facilities. That could improve operator safety and financial security associated with maintained production capacity and on-time delivery. A typical example of a potential risk is the improper connection of different tubing elements during bioreactor setup, media preparation, and inoculum transfer. An operator error during such critical operations might result in a leak, potentially causing contamination at a later time and loss of a run.
Scanning Different Test Methodologies
More than 40 different test methods have been proposed for leak detection (e.g., according to EN1779 “Leak testing: Criteria for Method and Technique Selection”) based on a number of technologies such as gas leak detection (integral or by probe), thermal imaging, flow measurement, and pressure decay and pressure increase.
Each technology has its own strengths and weaknesses in terms of
accuracy and reproducibility
rest time
handling aspects
condition of the bags after testing
possibility of testing connections
investment costs for equipment
cost per test
footprint of the test equipment
contamination risks
IP situation
feasibility of in-place testing (point of use).
Those factors have to be carefully considered and balanced in developing a suitable bag-testing device for single-use bioreactors for GMP production of vaccines, monoclonal antibodies, and recombinant proteins.
Few Methods Qualify for Postinstallation Preuse Testing
There is a risk of potential handling errors when installing single-use bioreactors into their holders. So a reliable leak test for such bags must be performed at the point of use (preuse but postinstallation). Testing a single-use bioreactor bag in a separate device (as is usually done when applying gas leak detection) and then installing it into its bag holder would not permit detection of operator-handling errors. It would merely compensate for potential shipping incidences. Also, the complexity and costs associated with gas leak detection technology would be unreasonable to make it a routine testing method. However, proper packaging (by the manufacturer) and inspection of the cardboard box (by the customer) would detect shipping incidences.
Mass or volumetric flow measurement is a well-known technology, but it requires a very precise pressure control throughout the test. Slightly overshooting or undershooting the initial test pressure would directly affect the measured flow and result in false estimation of the test value. The bigger the volume the more difficult it is to accurately adjust the pressure. This methodology therefore would not be applicable to larger volumes.
Pressure-decay technology, on the other hand, is a robust, easy to implement, cost-effective, and recognized technology that can be used to test a single-use bioreactor bag after installation in its final configuration directly in its holder. We have therefore used this approach to develop a reliable and predictive risk-mitigation tool.
Developing a Reliable and Reproducible Method
The objective of any test method must be to reliably identify potential damage of installed bioreactor bags covering bag seals, port welds, connections, and all bag surfaces. Any damage could result in a loss of bioreactor content or pose a biosafety risk.
We identified early on during the development of a method based on pressure decay that a leak in a plastic bag might be totally masked when pressed against a smooth, hard surface. Very little of the test gas can escape through the leak, and the reliability of the pressure-decay detection is nil. Therefore, performing pressure decay or flow measurement on a bag in a holder or even on a bag placed freely between two plates, without any porous spacer separating the bag from the flat surface, would not represent a reliable test set-up because of the masking effect.
Using a specifically designed, patent-pending polyethylene fleece acting as a porous spacer between the plastic film of the bioreactor and the holder prevents this masking effect (Figure 1). It delivers reliable and reproducible test values comparable to results obtained with the same test method on bag areas that are not covered by any hard surface.
Pressure-Decay Testing Methodology
The developed pressure-decay test method is based on the protocol from the American Society for Testing and Materials ASTM F2095-01 (3).The Sartocheck 4 plus Bag tester from Sartorius Stedim Biotech is used together with the above-described bag tester fleece that prevents masking of leaks that might have been potentially introduced during installation. It ensures reliable point-of-use leak testing of single-use bioreactor bags postinstallation and preuse in its final bag holder.
Pressure-decay measurement is very sensitive to temperature changes. Therefore, the Sartocheck 4 plus bag tester provides a temperature measurement of the environment and generates a warning message in case of excessive temperature drift during the test phase.
This pressure-decay leak test has been developed to reliably detect defects in BIOSTAT STR single-use bioreactor bags over the complete range from 50 L to 1,000 L. Qualification of the method at the 2,000 L scale is ongoing.

Figure 2: Point-of-use testing of singe-use bags
The bag is tested after installation, so potential leaks are detected in the bag walls, seals, or connections over the entire flexible bag system, including tubing. The test method is nondestructive and enables the implementation of a reliable and reproducible point-of-use test in bioproduction facilities (Figure 2).
The pressure decay during the test is measured and compared with acceptance criteria established during method qualification.
Qualification Limits Established During Initial Study
The validation approach consisted of two parts:
an engineering study to establish the detection limit for different bag volumes
formal qualification to verify the minimum detectable leak size and establish acceptance criteria of the test.
To determine the minimum detectable leak size, a simplified bag with only one bottom port connection for test gas application was prepared with multiple “defect patches” representing different defined leak sizes. A defect patch is a circular sheet of film with a laser-drilled and flow-calibrated leak diameter that was welded to the bag surface.
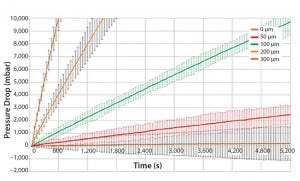
Figure 3: Engineering study results (BIOSTAT STR 200L bag)
For reliable detection of a given leak size there must be a significant difference in pressure decay between a bag with the given leak size and a bag without defects. By repeated testing, the standard deviation of the test value was calculated for the various leak sizes and for bags without defects. The minimum detectable leak size was defined to be the leak size of which the lowest pressure decay value was at least six times higher than the standard deviation of the highest pressure decay value of a bag without leaks.
Figure 3 shows results for different defect patch sizes for a 200 L bag volume. The pressure decay measured for a 50 μm defect patch was overlapping with a bag without leaks. Therefore, a 50 μm leak cannot reliably be differentiated to a bag without leaks. On the contrary, a 100 μm leak size shows a clear differentiation to a bag without leaks.

Table 1: Minimum detectable leak sizes for different Biostat STR bag volumes
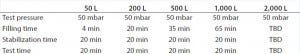
Table 2: Test program parameters for different Biostat STR bag volumes
In a second step, one standard bag without a defect patch and one standard bag with a defect patch of the previously determined minimum detectable leak size were used to establish the final test parameters. Table 1 shows the minimum detectable leak sizes for the different BIOSTAT STR bag volumes. Based on the results of the engineering study, the final test parameters for the qualification of the method were established (Table 2). Those parameters were used to generate a test program for each BIOSTAT STR bag volume using the Sartocheck 4 plus Bag tester.
Method Validation Confirms Reliable Detection of Leaks
During the final qualification study, the minimum detectable leak size for the different bag volumes was confirmed on a statistically significant number of standard bags from different routine production lots. Standard, nonmodified bags of different bag volumes and standard bags modified with a single defect patch representing the minimum detectable leak size were used. All bags were gamma irradiated. A minimum of 10 test repeats were performed per bag. Table 3 summarizes the maximum pressure decay measured for nondefect bags during the qualification study.

Table 3: Summary of maximum pressure decay for different bag volumes established during
qualification study
All tested bags showed expected results: The nondefect standard bags passed the test, and the standard bags prepared with a single defect failed. Hence, the test method using pressure decay combined with the fleece to prevent masking of leaks was successfully qualified and proven to be a robust and predictive method for reliable detection of leaks during routine operation.
Fast and Robust Bag Testing Method for Routine Use
This new, qualified single-use bioreactor leak test based on pressure decay using a specifically designed porous fleece enables reliable and robust routine postinstallation and preuse testing. With this novel test method, the same level of risk mitigation and assurance of operating procedures can be achieved for single-use bioreactors as previously achievable only with conventional stainless steel bioreactors that could easily be pressure-tested before use.
The pressure-decay approach is easy to implement on a routine basis. The automated testing sequence and user guidance ensure a low risk of operator-handling errors. Monitoring the environmental temperature during the full test sequence further enhances reliability of the test results and prevents false conforming and false nonconforming test results. The equipment is both robust and cost-effective.
One saved batch can provide an instant return on investment. The leak test helps to prevent derailing of production schedule and project delays. Especially in processes where biosafety is critical (e.g., vaccine production), it is an indispensable tool for protecting operator safety.
References
1 Chalk S, et al. Challenging the Cleanroom Paradigm for Biopharmaceutical Manufacturing of Bulk Drug Substance. BioPharm Int. Aug. 2011: 1–13.
2 EudraLex: The Rules Governing Medicinal Products in the European Union. Volume 4: EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use; http://ec.europa.eu/health/ documents/eudralex/vol-4/index_en.htm.
3 ASTM F2095-07: Standard Test Methods for Pressure Decay Leak Test for Flexible Packages with and without Restraining Plates; www.astm.org/Standards/ F2095.htm.
Corresponding author Magnus Stering is product manager, integrity testing solutions; Martin Dahlberg is manager, R&D instrumentation and control; Thorsten Adams is product manager, fermentation technologies, Davy De Wilde is director of marketing, fermentation technologies; Christel Fenge is vice president of marketing, fermentation technologies. All authors are from Sartorius Stedim Biotech.
You May Also Like






