The Dinosaurs Reborn: Evaluating Stainless Steel and Disposables in Large-Scale BiomanufacturingThe Dinosaurs Reborn: Evaluating Stainless Steel and Disposables in Large-Scale Biomanufacturing
December 1, 2010
Although a number of biomanufacturers have adopted disposable technologies for small-scale process design, there has been considerable debate over the role of single-use systems in large-scale biopharmaceutical manufacturing— particularly in retrofitting facilities. Some experts have gone so far as to suggest that large-scale stainless steel fermentors are “dinosaurs,” with their large capacities, long installation lead times, and low flexibility. I advocate a systematic approach to look holistically at possible retrofit technologies in existing (stainless steel) facilities, with particular reference to disposables.
My firm’s research suggests that in a retrofit setting, disposables have little proven value for reducing installation, materials, or labor costs. We instead look at such technologies in terms of their ability to reduce complexity in facility design and allow greatest flexibility in making changes to process design.
PRODUCt FOCUS: ALL BIOLOGICS
PROCESS FOCUS: PRODUCTION AND PROCESSING
WHO SHOULD READ: PROCESS DEVELOPMENT, MANUFACTURING, QA/QC, OPERATIONS AND FACILITIES MANAGERS
KEYWORDS: BIOREACTORS, SCALE-UP, BUFFER PREPARATION, FACILITY DESIGN AND ENGINEERING, LEACHABLES, EXTRACTABLES
LEVEL: INTERMEDIATE
Evolution of Disposable Systems
Single-use technologies have evolved quickly during the past decade, morphing from relatively simple bag-based solutions to increasingly sophisticated bioreactor and purification-based technologies. We do not seek to outline particular technologies available from vendors; good summaries are available (1,2). Figure 1 summarizes these data, showing the level of disposables adoption in existing large-scale facilities based on a cross-sectional survey of use across biomanufacturers.
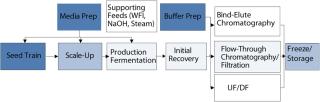
Figure 1: ()
Feasibility of Retrofitting Biomanufacturing Facilities
One issue arising from the current capacity abundance of stainless-steel units is identification of areas where disposables may benefit existing facilities. During the next 5–10 years, titers for new clinical and commercial processes will average >4 g/L, and some may reach 20 g/L (3). Many processes will be conducted in primarily stainless steel plants, which are already in operation or near completion. Even new facilities built in this period are likely to include stainless steel for production bioreactors and in much of the downstream purification train due to both technological issues and the risk adversity of the industry.
Because of ongoing improvements in titer and other plant operations, there is a growing excess of large-scale cell culture capacity and a need to understand how it is manifested in a specific plant setting. In many cases, retrofitting existing plants to handle high-titer processes can be done inexpensively and in short timeframes. However, looking at the feasibility of incorporating disposables technologies requires a more comprehensive analysis of the benefits of disposables than arguments presented for “green-fields” facilities.
Classic arguments for disposables highlight the reduced need for clean-in-place and steam-in-place systems, lower water for injection (WFI) use, and lower validation and qualification times (4,5,6). However, many facilities already have this support infrastructure in place, with highly evolved systems to reduce associated labor. WFI in facilities with already purchased (and partially depreciated) stills is also relatively inexpensive, at $0.05–0.20 per liter. In such cases, the benefits of retrofitting for disposables become less clear. The need to integrate them with existing conventional stainless steel systems requires setting priorities of key facility areas where disposables may provide benefit and can be implemented with minimal disruption. Here are four primary considerations.
Vessel Scale: The scale or sizing of a vessel under consideration is the first element in determining whether disposables are a suitable alternative to stainless steel. Many studies cite 2,000 L as the largest efficient size for current single-use vessels. Very few products are available for larger scales than this (2), mainly because of physical considerations: Full bags at or above the 2,000-L level are not self-supporting and are inherently unable to be transported without jack lifts (7). At 2 m3 in size, a 2,000-L vessel is difficult to fit down passageways, has significant momentum, and takes up considerable space.
Another factor is the potential market size for these “super-sized” bags. A relatively small number of biomanufacturers make batches of product, media, or buffers at such a scale, and the cumulative number of batches they produce in a year is low. So the overall demand for such bags is relatively small, offering little incentive for disposables manufacturers to produce them.
For 500- to 2,000-L batch sizes, fixed metal bins or other fixed containers hold the bags and prevent stretching or other plastic deformation. Their footprint is not dissimilar to the stainless steel technology being replaced (and associated considerations in terms of floor loading). Installation of these containment systems also requires engineering work, especially if multi-level bin systems are used to maximize available floor area.
It seems that with current technology, the most immediately beneficial applications of disposables come at scales ≤500 L (Table 1). Smaller scale disposables provide maximum flexibility as mostly turnkey solutions requiring little engineering consideration. The key advantage of such solutions is that they are mobile: They can be transported into a facility without engineering or interruption of production operations. This scale also provides the most flexibility for operations, allowing totes or bins to be moved as necessary between production runs to match needs in a particular area. At a small enough scale, the bins can even be moved while containing product, allowing operators to wheel materials from one area to another without the need for extensive piping.
Table 1: Tradeoffs of disposable hold vessels at various scales
>
Table 1: Tradeoffs of disposable hold vessels at various scales ()
Extractables and Product–Vessel Residency Times: Analysis of the impact of extractables and leachables on disposables implementation has received considerable focus and research investment (8). This is primarily an issue for single-use technologies that contact product, for which explicit validation and testing as well as supplemental data submission are often necessary. Some media and buffers are not well suited to particular bag technologies (typically because of pH or other considerations).
One primary high-level consideration here is the residency time of a product in a vessel at ambient or raised temperature. The longer the contact, the more consideration needs to be made of the effects of extractables, and the greater is the regulatory burden. (Many other factors are also important, including surface area of location in process, as well as the nature of the product and vessel materials.) Figure 2 represents the time spent by a product at each stage of a process. It suggests a “heat map” of disposables suitability, in which cooler colors represent relatively easy targets for validation of disposables technology, and warmer colors represent areas of increasing requirements for extractables and leachables studies.
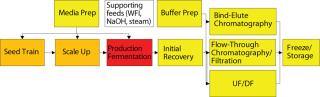
Figure 2: ()

Vessel Complexity: The next consideration for disposables-based manufacturing is the complexity of the vessel being replaced (Figure 3). Complexity has a number of meanings: the number of ingress and egress ports, the number of sensors required, and the level of process control required (e.g., impellers). One way to define vessel complexity is “anything that isn’t just the bag itself.” Ingress and egress ports are an obvious consideration for disposables because they alter the bag design and must be connected for each bag installation. Stainless steel, on the other hand, has hard-wired ports that are typically connected just once per campaign and have well-established methods for sampling and other regular sterile connections. This means “set-up” cost of a disposable vessel rises as a function of the number of ports that must be connected to the vessel.
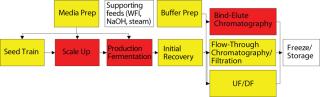
Figure 3: ()
Disposable-friendly measuring and sensor technologies are still a long way from their reusable counterparts and thus a critical issue for disposables implementation in complex vessels (9, 10). Because they must be installed and connected for each batch processed, these sensors are inherently less reliable than are fixed sensors. Current isposables-based sensors (e.g., multiuse noninvasive sensor technologies) are relatively expensive compared with stainless steel units, especially when calculating cost over the lifetime of a facility.
Process control also introduces complexity into tank design. Buffer and media hold tanks and (for most processes) downstream pool tanks are used solely for holding material between process stages. No mixing, agitation, or pressure control is typically required. Conversely, deep-tank biopharmaceutical reactors require a high level of process control to ensure uniformly distributed media, pH levels, temperature, and other optimal conditions for growth. The need to either design out an impeller or integrate a disposable impeller into a reactor or media/buffer preparation design adds complexity and additional cost. Some approaches to this (such as mixing by recirculation) are not as efficient as impeller methods and may lengthen the time needed to perform preparation processes (7).
The level of automation needed for process control is also an important factor when considering disposables implementation. A high level of process automation considerably reduces labor burden, particularly automated clean-in-place (CIP) systems. This minimizes the effect of labor savings due to disposables. Automation coupled with process control also involves sensing and responding systems that are typically hard-wired into tanks, columns, and other processing equipment. Implementing disposables typically allows a lower level of automation of key activities, because disposables-based technology is designed to be manufactured inexpensively and for a single use only. So disposable technologies are best suited for facilities with low levels of overall process automation control or where automation is less prevalent (e.g., the seed train or supporting operations such as media and buffer preparation).
Interprocess Complexity and Dependencies: When retrofitting a biomanufacturing facility, reducing interprocess complexity is important. Interprocess complexity is defined as any part of a process that is linked to more than one unit operation. There are numerous examples in stainless steel biopharmaceutical manufacturing facilities: tanks dedicated to media or buffer preparation/hold for more than one type of buffer; multipurpose pool tanks; CIP and SIP skids with the loops that support them; and (perhaps most important) common piping segments and transfer panels and valve arrays. These areas are typically the worst modeled and least understood parts of a manufacturing facility,
and they also are most associated with unforeseen bottlenecks and delays. Almost every biomanufacturing facility was originally designed for a higher run-rate than is actually practically possible. Interprocess complexity is frequently the reason why.
Figure 4 indicates the number of cross-connections by unit operation. In most biomanufacturing facilities, media and buffer preparation areas have the most interprocess complexity because they provide material to support multiple unit operations. Conversely, there is typically a clearly established path for main operations, transferring cell culture from one bioreactor to the next and through downstream operations. So a delay in batch processing has a clear effect on a facility because it affects only the next process step. This batch flow path typically has dedicated tanks for each processing stage, but buffer and media preparations (and sometimes hold tanks) are multiuse for a number of different buffers across different unit operations.
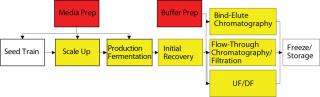
Figure 4: ()
Single-use technologies have exciting possibilities to reduce interprocess complexity and dependency between operations. Disposables reduce or eliminate the need for CIP and SIP processes, reducing a key dependency (and frequently a hidden bottleneck) in a facility. Removing these processes also eliminates much need for WFI drops and other piping that links together equipment. Flexible piping and disposable manifolds can also reduce the “interconnectedness” of piping segments, valve arrays, and transfer panels. Because such interconnected (or, more properly, “cross-connected”) parts of a facility are its least flexible aspects and areas where bottlenecks almost always occur, correctly “disjoining” a facility can significantly improve throughput.
A flow-on benefit for implementing disposables expressly to reduce the “interconnectedness” of a process is that it becomes much easier to analyze each unit operation independently and find critical bottlenecks. This makes it possible to retrofit a facility to allow for a new process with significantly less technical risk (because engineering changes are typically isolated to individual unit operations) rather than requiring new piping or additional support equipment to be installed.
Case Study 1: Disposables Technologies for Fermentation
A biomanufacturer was considering implementing disposables in an existing large-scale facility. Analysts had told the company that its key areas of focus for disposables should be hold bags and minimizing turnaround time for bioreactors, with a projected cost savings of >10%. The analysis aimed to confirm that savings and evaluate in greater detail the effect of implementing disposables for media preparation and in using disposables for parts of the scale-up process with bioreactors <1,000 L.
Media Preparation: To prepare media, the biomanufacturer used a number of tanks corresponding to each process class in scale-up. The process consisted of obtaining prekitted medium and adding it to the tank, then adding WFI from a WFI drop, adjusting pH in the tank, and transferring the media to a bioreactor. The media filter housing was also SIPed in a parallel process, and a filter was installed before the media were transferred to the bioreactor.
Figure 5 shows the percentage of time spent performing each activity for media preparation (idle time is ignored). Note that prep time for the disposable media bin includes time to move it to an appropriate location, as well as retrieving and installing the bag and connections to a production vessel. The biomanufacturer used recirculation methods to prepare media, which made mixing take several hours longer for disposable-based technologies. Media transfer also took longer for the disposable case because the tanks could not be pressurized to ensure a constant output flow. The final step involved cleaning up disposable assemblies and transfer lines, which is significantly shorter than an automated CIP process.
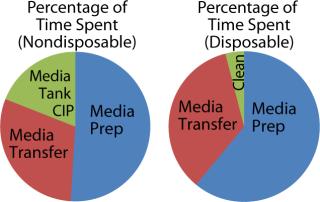
Figure 5: ()
This time and motion study indicated there is little direct time savings from switching to disposables technology for media preparation, provided that a single media preparation station is dedicated to each bioreactor. In addition, given the automated CIP process already established, the nondisposable option actually required less labor.
Installation of Disposable Bioreactors: Disposable bioreactors can eliminate the need for CIP and SIP operations, shortening the overall preparation time required for a bioreactor and maximizing its use. Figure 6 shows the time required to prepare a stainless-steel bioreactor at various scales in the facility (note the use of automated CIP and SIP systems). For disposable bioreactors, this creates potential time-savings of ~10–15 h (or ~10%) of the total scale-up and cycle time.
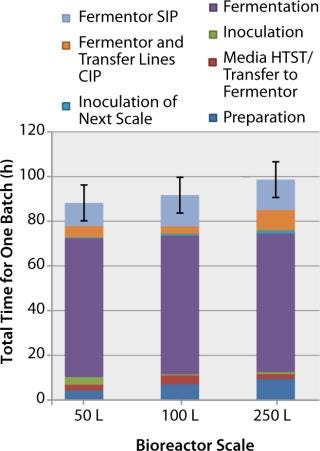
Figure 6: ()
To assess the effect of this 10–15 h savings, the biomanufacturer performed a sensitivity analysis with an existing model at its facility using Bio-G’s Simulation System. The model looked at the effect of these potential time savings in fermentation, as well as in other areas of the facility. Figure 7 shows the results of this analysis and the effect of reducing processing times for each activity in the facility to determine key bottlenecks. No areas in fermentation benefit from the cycle-time reduction. Critical facility bottlenecks at the titers considered are in the downstream Protein A column and the final ultrafiltration/diafiltration (UFDF) step. Although disposables considerably decrease turnaround time for bioreactors, such time savings cannot be realized in this facility because downstream operations cannot match the shorter upstream cycle time.
00000;” >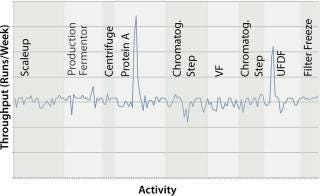

Figure 7: ()
Case Study 2: Disposables for Buffer Preparation and Hold
Subsequent to the analysis in Case Study 1, the biomanufacturer considered using disposables for buffer preparation/hold. A total of 14 buffers was needed for each batch processed. Buffer preparation tanks were shared between unit operations in a fixed pattern and cleaned using a CIP skid with an automated control system (no manual cleaning). Buffer hold tanks were both cleaned and steamed after use, again using an automated CIP and SIP processes.
For this case, several buffers needed to be prepared to support a single unit operation. So there was a considerably more compelling case for the use of disposables. Buffer preparation steps are typically associated with a high degree of variability in cycle time, reflecting involvement of shop-floor operators, a need for manual additions of several chemicals at precise volumes, and delays to allow for complete mixing as well as quality control testing in some cases. Figure 8 shows data indicating some of that variability, with successive preparations of the same buffer taking 1.2–6.0 h.
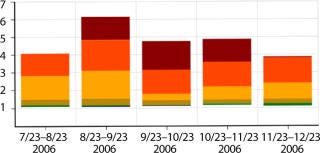
Figure 8: ()
The variability seen in buffer preparation times created issues with cleaning and steaming the tanks between cycles as well as resource contention issues with the CIP and SIP skids for cleaning other tanks and pieces of equipment.
Figure 9 shows the effect of switching to disposables for buffer preparation (using open liners) and for both buffer preparation and hold tanks in all areas of the downstream purification train. The graph shows two main benefits, neither of them immediately obvious to engineers. The first benefit is a slight increase (5–10%) in the facility’s average run rate. This is due to a decrease in CIP and SIP skid use — making those skids available to clean key bottleneck pieces of equipment such as chromatography skids and product hold vessels. The second benefit is a decrease in the overall variability of the time to produce a batch, leading to more reliable facility use (and flow-on benefits for overtime and scheduling). In this case, the standard deviation of the run rate decreased from 0.2 runs per week to 0.08 runs per week (as indicated by the narrower range of values in the histogram). A smaller standard deviation means that the facility is more likely to run on schedule, producing a highly reliable number of batches per week rather than a wide range of possible values.
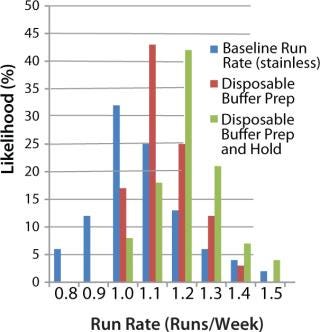
Figure 9: ()
A Place for Stainless Steel
The benefits of disposables for building green-field biomanufacturing facilities is clear and relatively simple to analyze. Such facilities are always “right sized,” and reduced installation and validation times offer significant benefits in facility design and construction. The advantage of retrofitting large-scale facilities with disposables is less obvious and requires much more detailed analysis by simulation or other such tools. Key benefits in a retrofit case are the ability for disposables to reduce inter-process complexity and “disjoining” a facility into a simple collection of unit operations with little or no dependencies. Coupled with a focus on critical facility bottlenecks — particularly in areas where simple bag-based solutions can be installed with little or no engineering or validation work — offer the most significant potential to allow stainless steel “dinosaurs” to be reborn as nimble, agile, and flexible facilities for decades to come.
About the Author
Author Details
Rick Johnston, PhD, is principal at Bioproduction Group Inc.; 1-510-704-1803; [email protected]; www.bio-g.com.
REFERENCES
1.) Zheng, R. 2010. The Game Changer. BioProcess Int. 8:S4-S9.
2.) Anicetti, V. 2009. Biopharmaceutical Processes: A Glance into the 21st Century. BioProcess Int. 7:S4-S11.
3.) Croughan, G. 2010.Beyond Just High Titers: The Future of Cell Line Engineering, IBC Cell Line Development and Engineering Conference, San Francisco.
4.) *Gottschalk, U. 2009. Disposables in Downstream Processing. Adv. Biochem. Eng. Biotechnol. 115:171-183.
5.) Eibl, R. 2010. Disposable Bioreactors: The Current State-of-the-Art and Recommended Applications in Biotechnology. Appl. Microbiol. Biotech. 86:41-49.
6.) *Sinclair, A, and M. Monge. 2009. Disposables Cost Contributions: A Sensitivity Analysis. BioPharm Int. 22:14-18.
7.) Ravise,. 2009. Hybrid and Disposable Facilities for Manufacturing of Biopharmaceuticals: Pros and Cons. Adv. Biochem. Eng. Biotechnol. 115:185-219.
8.) Bestwick, D, and R. Colton. 2009. Extractables and Leachables from Single-Use Disposables. BioProcess Int. 7:S88-S94.
9.) Rao, G, A Moreira, and K. Brorson. 2009. Disposable Bioprocessing: The Future Has Arrived. Biotechnol. Bioeng. 102:348-356.
10.) *Bernard, F. 2009. Disposable pH Sensors. BioProcess Int. 7:S32-S36.
You May Also Like






