- Sponsored Content
Reduce Cost, Increase Quality, and Shorten Cycle Time in Upstream Manufacturing with a Single-Use Heat ExchangerReduce Cost, Increase Quality, and Shorten Cycle Time in Upstream Manufacturing with a Single-Use Heat Exchanger
August 15, 2014
 Successful cell culture on a large scale requires close control of the culture environment in terms of temperature, pH, removal of waste products, and addition of fresh nutrients. To produce high yields of a desired protein, precise control over such parameters at every step in the process is required. Additionally, FDA regulations require various steps to be taken to control bioburden and maintain sterility. These controls and regulations in cell culture environments have led to an increasing move toward single-use systems with the goal of achieving reduced cleaning requirements, high levels of sterility, and zero batch-to-batch contamination (1).
Successful cell culture on a large scale requires close control of the culture environment in terms of temperature, pH, removal of waste products, and addition of fresh nutrients. To produce high yields of a desired protein, precise control over such parameters at every step in the process is required. Additionally, FDA regulations require various steps to be taken to control bioburden and maintain sterility. These controls and regulations in cell culture environments have led to an increasing move toward single-use systems with the goal of achieving reduced cleaning requirements, high levels of sterility, and zero batch-to-batch contamination (1).
Single-Use Solutions
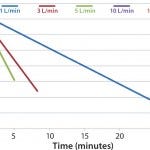
FIGURE 1: DHX cooling efficiences measured by temperature vs. time
Because of contamination concerns, single-use bag systems have gained widespread acceptance and are commonplace in biopharmaceutical manufacturing. More recently, single-use bioreactors for cell culture, single-use tangential-flow filtration (TFF) systems for harvest, and disposable options for downstream processing are being adopted (1, 2). However, without an available high-capacity, single-use heat exchanger, manufacturing engineers have been forced to choose one of two less than ideal options:
Insert a stainless steel multiuse heat exchanger into the process. This sacrifices the benefits of a full single-use set up resulting in prolonged downtime and expensive capital equipment.
Use a jacketed mixing tote in place of a heat exchanger with a sacrifice in terms of heat transfer, because the jacketed mixing tote was never designed for rapid temperature control.
Those solutions are not ideal for every application. Each has brought a series of problems for which manufacturing engineers have had to contend.
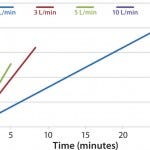
FIGURE 2: DHX heating efficiences measured by temperature vs. time
The DHX single-use heat exchanger offers significant advantages for cell culture at the manufacturing scale, allowing manufacturing engineers to build temperature control into their process without having to sacrifice the benefits of single-use. For the first time, it is possible to add temperature control into the cell culture process flow while retaining a complete single-use set up enabling 100% confidence in sterility while increasing the quality of finished product through uniform temperature control.
References
1. Shukla, A., Gottschalk, U. Single-Use Disposable Technologies for Biopharmaceutical Manufacturing,
Trends Biotechnol 2012; 31 (3), 147-154.
2. Jones, S. Single-Use Products for Bioproduction: Available Options for Cell Culture and Downstream Processing
Am. Pharma. Rev. 1 May 2012
Author
Niraj Chandarana is director of product development life sciences, 163 Research Lane, Millersburg, PA 17061; 1-717-692-2104;
[email protected]
You May Also Like






