The biopharmaceutical industry is continually reinventing itself. It evolved from a science-driven entrepreneurial approach and has worked over five decades to weave its network of business, regulatory, and process-development modes somewhat after the fact. The origin of so many bioprocesses in academic research laboratories prompted some of us in the editorial world to propose article series on “biobusiness 101 for scientists” back even in the early 1990s. Much of the industry’s progress toward its current form depended on adapting processes from the classical pharmaceutical and other sectors. Borrowing approaches from other long-established industries — e.g., chemical, semiconductor, automotive, and food — is an excellent way to prevent reinventing various wheels. But those wheels still have to be adapted for the needs of new vehicles and can end up doing a lot of spinning before they gain traction.
A number of the issues with which the current industry is struggling are mentioned and alluded to in this...
Illustration of a pancreas gland with islets
ADOBE (
STOCK.ADOBE.COM
)
During the January 2023 Advanced Therapies Week in Miami, FL, Leonardo Ferreira, an assistant professor from the Medical University of South Carolina, spoke about his team’s work in developing chimeric antigen receptor (CAR) regulatory T cells (Tregs) as living therapeutics for type 1 diabetes. Ferreira has focused on type 1 diabetes since 2016, when he undertook postdoctoral work at the University of California at San Francisco under Qizhi Tang and Jeff Bluestone before continuing his research at his own laboratory in Charleston, SC. He spoke to BPI about his lab’s progress toward developing a therapy for type 1 diabetes in a two-part interview. In next month’s Part 2, Ferreira will discuss the characteristics that make CAR Tregs a good choice for treating Type 1 diabetes.
The Biology of Type 1 Diabetes
Can you tell us about type 1 diabetes and how it affects those who have the disease?
Type 1 diabetes is a devastating autoimmune di...
A Theradaptive scientist works with a three-dimensionally printed construct that will carry material-binding therapeutic proteins for bone-tissue regeneration.
In March 2023, I took the opportunity to speak with Luis Alvarez about the founding of Theradaptive. The company specializes in engineering recombinant proteins, with an initial focus on developing therapeutics for regeneration of soft, vascular, and bone tissue. “Theradaptive grew out of my thinking about combat injuries,” Alvarez told me. Before earning a doctoral degree in biomedical engineering from the Massachusetts Institute of Technology and working as cofounding deputy director of the US Department of Defense’s regenerative medicine program, he served in the US Army during the Iraq War.
Alvarez witnessed severe injuries during his tour. “When I returned from Iraq,” he continued, “I spoke with friends who were physicians at the Walter Reed National Military Medical Center in Bethesda, MD. They told me about their experiences in performing
d...
ADOBE (
STOCK.ADOBE.COM
)
Shifting to a digital regulatory environment is forcing pharmaceutical companies to confront knowledge gaps across key research and development (R&D) functions. As health authorities streamline information exchange through data standardization, the separation between regulatory operations and other functions within the pharmaceutical industry begins to blur. By advancing implementation of ISO identification of medicinal product (IDMP) standards, companies are improving interoperability and information sharing among key clinical, pharmacovigilance, quality, manufacturing, and supply-chain–logistics teams. However, a company’s important regulatory and strategic planning information often is found in paper documents, bogging down the organization’s data value chain.
The need to prepare for new digital regulatory-submission requirements can present obstacles for companies. European Medicines Agency (EMA) requirements include adherence to the InterSystems IRIS platform for reporting m...
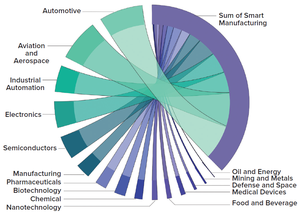
Biopharma 4.0
refers to applications of data and digital technologies to biotherapeutic manufacturing. Technological advances now enable the internet and its embedded systems to serve as a nucleus through which biomanufacturers can integrate production lines and processes across organizational boundaries, thereby forming a networked and agile value chain. Solutions under the industry 4.0 umbrella include
• platforms for “smart” manufacturing made possible by the internet of things (IoT)
• artificial intelligence (AI)
• systems for process automation
• technologies for remote operations
• systems for agile manufacturing.
However, a barrier to industry 4.0 adoption is emerging. The skills and expertise that are required to operate such technologies are misaligned with the available talent pool.
The Evolution of Biomanufacturing Talent
Rapid expansion in the biomanufacturing industry has become a key driver of the biopharma 4.0 paradigm. Transitioning to that approach requires a workforce that can succeed i...
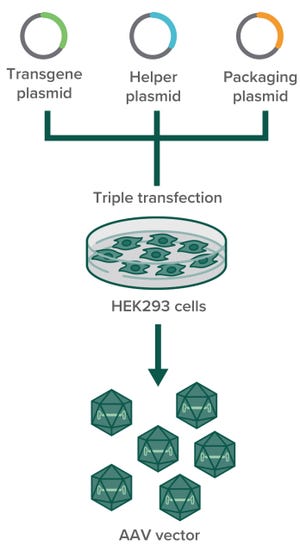
Adenoassociated viruses (AAVs) have been the center of intensive research since their fortuitous (incidental) discovery in 1965 as a contaminant in a simian adenovirus preparation (
1
). Initial scientific interest primarily focused on understanding the fundamental biology of this virus type, but later it was harnessed to serve as a genetic vector for use in treating or even curing certain genetic diseases (
2
). Many distinct characteristics make AAVs a versatile tool for development of clinical candidates, exemplified currently by more than 260 clinical trials in progress for products manufactured using this delivery platform (
3
).
The safety, broad tropism, and ease of genetic engineering with AAVs are advantageous features of the platform. Despite substantial scientific and industrial interest in the AAV vector, however, large-scale manufacturing remains a major challenge in the field.
To optimize AAV manufacturing, we first must understand the biology of the virus. As indicated by the name of the ge...
Quantitative Risk Assessment of Limits for Residual Host-Cell DNA: Ensuring Patient Safety for In Vitro Gene Therapies Produced Using Human-Derived Cell LinesQuantitative Risk Assessment of Limits for Residual Host-Cell DNA: Ensuring Patient Safety for In Vitro Gene Therapies Produced Using Human-Derived Cell Lines
HTTPS://ADOBESTOCK.COM
Viral-vector gene therapies (GTs) manufactured from cell-substrate production systems can contain residual amounts of host-cell DNA (hcDNA), which in a final product presents safety risks to treated patients. Therefore, drug manufacturers monitor and control residual hcDNA levels in purified products (
1, 2
). The US Food and Drug Administration (FDA) and other global regulatory agencies recommend tight, quantifiable limits for hcDNA levels: 10 ng/dose, with DNA fragments smaller than the functional gene size of 200 base pairs (bp) (
3
). However, because of the inherent nature of hcDNA encapsidation in viral capsids, the FDA permits GT manufacturers to minimize the amount and size of hcDNA based on risk assessment rather than requiring quantifiable hcDNA limits. Thus, manufacturers define their own hcDNA specification limits for final products based on risk assessments to ensure patient safety (
4
).
Such assessments are not necessarily straightforward, and they require methods and...
Figure 1:
Containers in the Celsius platform are scalable to 75 L.
Bulk drug substances (BDS) often require frozen storage to preserve the integrity of biological material. Cold-chain management is particularly important given recent trends toward increasing globalization and decentralization and diversifying modalities. A secure cold chain is essential for maintaining a product’s critical quality attributes (CQAs) — such as drug product stability, integrity, and purity — and to prevent costly product losses.
Scaling Up the Cold Chain
Frozen storage at commercial scale presents its own challenges; facilities must be able to handle large volumes of BDS without jeopardizing product CQAs and operator safety. Currently, manufacturers have two main choices for storing and shipping large volumes of frozen materials:
• using large-scale, multiuse stainless-steel freeze containers
• multiplying the number of small-/medium-scale containers (single-use containers or bottles).
Stainless-steel containers are robust ...
The specialized nature of cell and gene therapies (CGTs) requires that they be delivered to single patients or in low batch numbers. Manufacturing CGTs at scale is critical to industry success, but doing so at an economically viable cost is a key obstacle. Today’s therapies cost between US$400,000 and $3.5 million per patient, largely because of the need for highly skilled workers to deliver those innovative drugs. The CGT industry has innovated across all areas of production, from collection of patient material to autologous manufacturing.
Strengthen Data Management and Integrity
Data integrity continues to be a priority area for good practice (GxP) inspections. Deadlines for replacing noncompliant record and data-management systems have passed, and as a result, organizations in late-phase advanced therapy investigational medicinal products (ATIMPs) and commercial manufacturing are obliged to operate electronic systems. Among further points of consideration for the CGT sector, two unique identifying code...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.