Gene therapies hold great promise for one-time treatments that alleviate or cure certain genetic conditions. Recombinant adenoassociated viral (AAV) and lentiviral vectors have emerged as leading gene delivery methods for in vivo and ex vivo gene therapies, respectively. With more than 500 gene therapy clinical trials underway, the industry needs scalable, cost-efficient viral vector manufacturing.
To address those needs, Corning developed the Ascent fixed-bed bioreactor system for scalable, high-density, adherent cell culture, including high-yield viral vector manufacturing. The Ascent fixed-bed bioreactor system includes a specially treated and packed polymer mesh that enables uniform low-shear fluid flow through the bioreactor bed. The system also provides homogenous cell attachment and growth, >90% transfection efficiency, and a significantly higher AAV vector yield/m
2
than other fixed-bed reactors. Furthermore, the ability to harvest viable cells from the Ascent system enables its use in seed train...
Figure 1: Observer validation
In bioreactor processes, the on-line monitoring of key parameters relies on the use of adequate sensors. In this article, we implement model-based software sensors to monitor nitrate concentrations in an algae batch process. Nitrate concentration is a crucial component in algae growth, and its assessment is used to achieve optimal growth conditions or to determine optimal harvest times. Batch cultivation of the algae
Chlamydomonas reinhardtii
is carried out in a Labfors 5 airlift photobioreactor provided by INFORS-HT. By means of an extended Kalman–Filter observer scheme, the software sensor is embedded in an eve software platform, which offers a communication structure with the Labfors reactor and enables an operator to implement real-time code for monitoring.
Fill out the form below to read the complete technology review and learn more about the eve software platform now.
Biopharmaceutical manufacturing is evolving with the progression of Industry 4.0. Industry 4.0 refers to the ongoing “fourth industrial revolution,” which is transforming modern manufacturing and production practices through the use of “smart” technology. This is appealing especially to the biopharmaceutical industry, where production can be a long, meticulous, complex process, and optimizing manufacturing procedures is critical for success. As a leading supplier of single-use technology for the biopharmaceutical industry, PendoTECH recently has explored how its products can be integrated easily with an industrial platform and the opportunities that result.
At the heart of the technology that enables PendoTECH sensors and instrumentation to be adapted conveniently to a digital highway is an intelligent automation platform (IAP). Typically, an IAP has analog inputs and digital inputs/outputs for communicating with all unit-operation devices essential to manufacturing (e.g., sensors, transmitters, and monit...
Figure 1: Model clinical virus production is increased in STAT1 KO cell lines with either (left) Influenza A or (right) Dengue II virus.
Antiviral vaccines are essential for preventing epidemic disease. However, production of them often is limited by low-yielding manufacturing processes. Similarly, the development of gene therapies is constrained during the production of adenoassociated virus (AAV) delivery platforms for gene transfer. To address the need for efficient viral synthesis to quicken the pace of bioproduction, ATCC used CRISPR/Cas9 genome-editing technology to develop STAT1 and BAX knockout (STAT1 BAX KO) cell lines to produce high-titer viral stocks. Three cell lines optimized for virus production were developed from parental cells commonly used in virus manufacturing. Those newly created cell lines can produce clinical viruses and AAVs at titers that are much higher than those of the parental cell lines, thereby providing an inexpensive method for biopharmaceutical companies to expand produc...
Avid Bioservices, Inc. is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on biopharmaceutical drug substances derived from mammalian cell culture. Avid’s services include process development and current good manufacturing practice (CGMP) clinical and commercial drug-substance manufacturing, bulk packaging, release and stability testing, and regulatory submissions support. For early stage programs, the company provides process development activities such as upstream and downstream development and optimization, pilot-scale manufacturing, analytical methods development, and testing and characterization. Avid has 28 years of experience producing a comprehensive range of proteins, including monoclonal antibodies (MAbs), recombinant proteins, bispecific monoclonal antibodies (bsAbs), Fc-fusion proteins, and biosimilars. The scope of our CDMO services ranges from stand-alone process development projects to full development and manufacturing programs through to commer...
Advancements in life sciences technologies and the increasing demands for improved, effective, and innovative products to prevent, treat, and in some cases, cure diseases have focused the attention of drug developers on new modalities such as mRNA-, DNA-, viral vector-, and cell-based therapies and vaccines. Novel biopharmaceutical products bring a level of complexity to developing and bringing them through design, clinical studies, and to the market. Like traditional drug products, complex therapies require robust, scalable manufacturing processes, a reliable quality-driven supply chain, demonstrable process control, and adherence to regulatory guidelines from upstream through the downstream and fill–finish processes.
Fill out the form below to read the complete capabilities review and learn more about Catalent’s approach to manufacturing novel biopharmaceutical products now.
Figure 1: Human mesenchymal stem cell (hMSC) viability using an X-WASH system
Cryopreservation is a necessary part of workflows for both autologous and allogeneic therapies. The ability to cryopreserve cells for cell therapy increases the range of administration, shelf life, and time for safety testing. Cryoprotectants such as dimethyl sulphoxide (DMSO) often are added to freezing media to increase post-thaw cell survival. However, DMSO itself can be cytotoxic, so reducing its final concentration can be necessary. Below, I demonstrate how the Corning X-WASH system can reduce the amount of DMSO used in cryopreserved cells through a semiautomated, closed system. Using the Corning X-WASH system, we reduced DMSO concentration significantly for cryopreserved bone marrow-derived human mesenchymal stem cells (hMSC) while maintaining high cell recovery and viability.
Fill out the form below to read the complete technologies review now.
Traditional cleanroom infrastructures, gypsum, or monolithic wall panels have been used in the past with varying success and benefit. One of the most often proclaimed benefits is the cost of those on-site built structures. Characteristically, the cost quoted at the beginning of a construction project seems to be attractive, but construction estimates are not better than ±50% at feasibility (preconcept) and ±25% at end of conceptual design. Given the above accuracy ranges, industry surveys establish that in most cases, the cost proposal is not met with traditional builds. Those types of cost-control variations cause major problems with budgeting and funding projects. That is because the funding value is either inflated to allow for cost variation (with the risk funding not approved) or another round of funding is needed mid-project. Both are undesirable scenarios. Off-site prefabricated cleanroom infrastructures enable robust cost budgeting because the resource and material loads are well defined and known...
Oxgene, a WuXi Advanced Therapies (WuXi ATU) company, provides cell and gene therapy pioneers around the world with technologies, automation platforms, and service solutions to advance their preclinical research and accelerate their timelines to good manufacturing practice (GMP) manufacturing. WuXi ATU, a global contract testing, development, and manufacturing organization (CTDMO), offers world-class, integrated GMP manufacturing and testing platforms to reduce time to market, while maintaining high titers, high levels of quality assurance, and full regulatory compliance. Together, our complete end-to-end cell and gene therapy solutions support pioneering companies to be first in the race to deliver breakthrough therapies to patients.
Fill out the form below to read the complete capabilities review and learn more about end-to-end cell and gene therapy solutions now.
Richter-Helm BioLogics is a leading and steadily growing contract manufacturer based in Germany. We comply with good manufacturing practice (GMP) and specialize in microbial biopharmaceutical products. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Clients worldwide already have benefited from our commitment to GMP, flexibility, and total transparency. With a proven 30-year track record and highly skilled professionals, Richter-Helm supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and qualified-person (QP) release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L. Two additional bioreactors with capacities of 300 L and 1,500 L for microbial production will be added. That includes establishment of mid- and downstream processing and supporting utilities. By end of 2023, the new trains will be fully operational.
Fill out the form belo...
Frozen storage of bulk drug substances (BDS), including bioprocess solutions, vaccines, blood components, and other delicate process fluids is common in the bioprocess industry. Vessels used to store those fluids must be capable of withstanding long-term storage in extremely cold temperatures (e.g., –85 °C or –196 °C), sometimes for very long time periods. They also must also maintain integrity after repeated thawing and subsequent refreeze. The study below was performed to characterize performance of fluoropolymer bottles with standard and two-piece closure systems after multiple freeze–thaw cycles, with a retorque step added after each freeze cycle.
Fill out the form below to read the complete technology review and learn more about the freeze–thaw performance of fluoropolymer bottles now.
Sponsored Content
Chimeric antigen receptor (CAR) T cell therapies have advanced rapidly in recent years, with a number of targets in clinical research and several US Food and Drug Administration (FDA)-approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells.
Cell isolation, gene editing, expansion, and cryopreservation are complex steps in a typical autologous CAR T cell manufacturing process. Integrating this complicated multistep workflow into a closed, modular, benchtop system can facilitate transitions from laboratory scale to clinical manufacturing and improve a CAR T cell product’s consistency, purity, and safety. Thermo Fisher Scientific presents a digitally compatible, good manufacturing practice (GMP)-compliant, semiautomated manufacturing platform, which when us...
With the rapid advancement of digitalization, new technologies and flexible production concepts have become essential parts of the biopharmaceutical industry, especially for implementing Industry 4.0, the Internet of things (IoT), and predictive maintenance. As an expert partner for digitalization, Zeta supports its customers with new tools for smart maintenance.
Swift commissioning helps biomanufacturers complete their projects successfully. That requires trained personnel and maintenance support. Intelligent tools based on digital plant data ensure smooth operation. Smart Maintenance Navigator (SMN) technology is a best-in-class example. It was developed to meet production-facility requirements at leading pharmaceutical companies. Smart Maintenance Navigator technology runs on digital end devices (e.g., tablets, smart phones, and wearables) and enables maintenance staff to access all information relevant for maintenance easily at any time and place, thus saving valuable downtime.
Fill out the form below...
Figure 1: Viable cell volume (VCV) and viability data for HIP CHO in the HyPerforma 3-L Glass Bioreactor and 50-L DynaDrive Single-Use Bioreactor cell runs
At least one report estimates that by 2025, 35% of biologics will be manufactured using enhanced processes, particularly perfusion-based bioprocessing. Results of two innovations that support this need for enhanced processes are examined below.
The Thermo Scientific HyPerforma DynaDrive Single-Use Bioreactor (SUB), the latest advancement in SUB technology, offers better performance than other bioreactors and scalability up to 5,000 L. Gibco High-Intensity Perfusion CHO Medium is formulated to provide exceptional performance and ease of use, with capability of sustaining >1 g/L/day continuous productivity, >100 x 10
6
/mL cell density, and 95% viability at one vessel volume per day (VVD).
Fill out the form below to read the complete technology review and learn more about HyPerforma DynaDrive and Gibco High-Intensity Perfusion CHO medium now.
Purification of recombinant proteins remains a critical challenge for applications ranging from basic biological research to the development and production of lifesaving biopharmaceuticals. At laboratory scales, the rapid purification of large numbers of new and uncharacterized protein targets effectively compels the use of affinity tags, which enable reliable purification using simple, established protocols with minimal optimization. Tags cannot be used for therapeutic applications, however, because of their potential for immunogenicity. Thus, for proteins other than monoclonal antibodies (which typically use a protein A affinity platform), biopharmaceutical manufacturing processes must rely on multiple conventional chromatography steps, with six months or longer being spent on developing an acceptable downstream process for a single, well-characterized target. The need for such contrasting approaches has led to the emergence of two worlds in protein purification: one in which affinity tags are used to a...
Figure 1: Cygnus AAE-MS contract services
Cygnus Technologies, part of Maravai LifeSciences, is the biopharmaceutical industry’s partner in host cell protein (HCP) and other process-related impurity detection and analytics. In addition, Cygnus now provides innovative viral clearance solutions.
Cygnus helps companies developing therapeutic proteins, vaccines, antibodies, plasma derivatives, and gene therapies ensure the safety of their biotherapeutics before human trials, regulatory approval, and commercial release. Cygnus provides analytical tools and solutions for improving bioprocess development for faster regulatory approval and better clinical outcomes.
Cygnus is an industry pioneer responsible for developing and commercializing the first generic assay kits for HCP detection. Its reputation for quality is recognized by the industry and global regulatory agencies. Cygnus continues to advance the science of bioprocess impurity detection with new breakthroughs, including its antibody affinity extraction ...
There is an established, global pipeline of existing and upcoming monoclonal antibody (MAb) drugs that treat a wide variety of clinical indications. In MAb manufacturing, protein A chromatography is a proven downstream purification process, but there remains a need to reduce total costs while improving purity and yield. The Avantor J.T.Baker BAKERBOND PROchievA recombinant protein A chromatography resin advances the production of MAbs by providing different choices to biopharmaceutical supply chains. Offered both as a bulk resin and as prepackaged laboratory columns, the BAKERBOND® PROchievA resin is suitable for purifying MAbs, FC fusion proteins, and bispecific antibodies. The high-performance resin enables efficiency in workflow processes and protocols.
Fill out the form below to read the complete technology review now.
Sponsored Content
Scalable, Real-Time Bioprocess Monitoring Solution: Kaiser Raman Technology and Sartorius BioPAT Spectro Yield Value from Early Process Development to Single-Use ManufacturingScalable, Real-Time Bioprocess Monitoring Solution: Kaiser Raman Technology and Sartorius BioPAT Spectro Yield Value from Early Process Development to Single-Use Manufacturing
Raman spectroscopy is used in biomanufacturing as a process analytical technology (PAT) tool for making rapid, nondestructive, in-process measurements. However, Raman data collection at early stages of bioprocess development has been a challenge because of the lack of interface to bioreactors <250 mL. Realizing the industry was struggling to capitalize on the full potential of Raman spectroscopy, Kaiser Optical Systems, Inc. (Kaiser), an Endress+Hauser company, collaborated with Sartorius to bring Raman to Ambr 15 microbioreactors (10–15 mL) and Ambr 250 minibioreactors (100–250 mL). Sartorius’s new BioPAT Spectro platform enables Kaiser Raman–based quality by design (QbD) early in process development and scales up to Biostat STR single-use bioreactors.
The following Q&A explains how Kaiser Raman technology and the BioPAT Spectro platform by Sartorius work together to provide biopharmaceutical manufacturers with scalable, real-time bioprocess monitoring.
Fill out the form below to read the complete capabi...
Novo Nordisk Pharmatech’s high-purity, nontherapeutic insulin is sourced directly from the Novo Nordisk parent company, the world’s largest insulin producer. The product consists of insulin human crystals that are biosynthetically produced by recombinant microbial expression in yeast.
Insulin human AF stimulates the proliferation of cells and enhances the yield, and it is a key component in serum-free growth media for mammalian cells. Insulin human AF is used for manufacturing monoclonal antibodies, virus vaccines, gene therapies, and other biological drug products approved by regulatory agencies worldwide, including FDA and EMA.
Safeguarding Your Regulatory Compliance
If you manufacture products for a market outside of your own, ensuring regulatory compliance can be particularly difficult. You must stay up to date with changes, navigate language barriers, and meet requirements above the official guidelines. Our one-stop regulatory support provides you with everything you need quickly and conveniently. Wh...
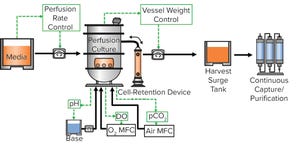
Figure 1: Typical configuration of WuXiUP platform
Recent world events have demonstrated now more than ever the growing demand for pharmaceutical biologics that can be made rapidly and in high volumes yet somehow remain affordable. Hence, there is an urgent need to develop a next-generation biomanufacturing solution that provides high-yield, high-quality drug products and is highly flexible and cost-effective. Herein we describe the WuXi Biologics ultrahigh
productivity platform (WuXiUP), an intensified perfusion culture process developed to meet the aforementioned need. WuXiUP adopts process-intensification strategies on to traditional perfusion culture processes to boost cell density and cell-specific productivity. The continuous harvest reduces greatly the residence time for a product within a bioreactor, leading to more desirable product quality and facilitating integrated continuous bioprocessing.
Fill out the form below to read the complete technology review now.
Figure 1: Capabilities flow chart
As a result of increased focus on customized medicine and continued emphasis on drug
development for rare diseases, the biopharmaceutical industry continues to advance its methods for disease treatment by developing target-specific novel therapeutics.
Formulations for these types of programs are complex and therefore present challenges in the design, scale-up, and manufacture of drug substances and drug products. Such challenges can delay getting critical products to patients. Contract development and manufacturing organizations (CDMOs) can help advance therapeutics and navigate production hurdles that traditionally can slow those programs down.
Ajinomoto Bio-Pharma Services is your trusted CDMO partner, providing a broad range of capabilities, regulatory excellence, and extensive experience to help you navigate those challenges, provide solutions to your development process, and deliver your new therapies to patients who need them.
Fill out the form below to read the com...
byJB Agnus
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.