January-February 2022
Happy 2022 to all of BPI’s loyal and supportive authors, readers, and advertisers. Despite continuing pandemic impediments, your industry continues to show remarkable flexibility and resilience. As technologies evolved to meet the demands for vaccines, tests, and treatments for COVID-19, new hybrid options have opened up opportunities for people to meet and collaborate.
Given the changing fortunes of our own publishing industry, managing this hybrid, controlled-publication model (peer-review + trade/B2B content) requires some agility of our own. So it should not be surprising that we editors are eager to celebrate every milestone. This year is BPI’s 20th year in publication (thus the special icon you’ll see on our cover throughout 2022), so we have a number of projects in the works and ways for you to help us celebrate.
BPI was founded in May 2002 (through Informa’s IBC conference group), with our first issue appearing in January of 2003. The founding staff included publisher Brian Caine, associate publis...
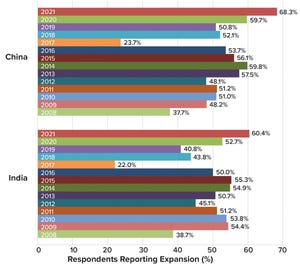
The global biopharmaceutical industry had been growing robustly even before the COVID-19 pandemic. According to the BioPlan Associates
Top 1000 Biofacility Index and Biomanufacturers Database
(
1
), bioprocessing capacity worldwide increased an average of 12% over the past decade. China has seen nearly double that rate. India’s bioprocessing segment also is showing strong growth. Because those regions represent 37% of the world’s population — and with rapidly growing middle-class economies, — demand for biologics there is outstripping that elsewhere.
Table 1:
Global comparison of biomanufacturing capacity; data from the BioPlan
Associates
Top 1000 Biofacility Index and Biomanufacturers Database
(CAGR =
compound annual growth rate).
Historically, Western technologies, equipment, reagents, and consumables have been used to produce biologics in China and India. Now, with COVID-19 disrupting international supply chains, domestic suppliers in those regions have begun to take on more important roles than e...
HTTPS://STOCK.ADOBE.COM
An innovative biopharmaceutical product can transform from an abstract idea at small scale into the basis of a burgeoning startup company. At that point, company leaders seek ways to ensure that a biologic will scale up in a quality-controlled, professional, and sustainable environment. That involves refining a research-stage prototype into a product that will be consistent and reproducible for research and development (R&D) and manufacturing and that will meet all relevant regulatory standards in specified target markets. Within the constraints of a small, early stage company with limited resources, however, making such refinements might be difficult or impossible.
To develop a product that meets the above standards, biomanufacturers are required to demonstrate critical quality attributes (CQAs) and critical process attributes (CPAs) for their manufacturing processes. Those concepts provide data-rich frameworks that can be monitored and maintained to ensure that end-product safety...
HTTPS://STOCK.ADOBE.COM
The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (
1
). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (
2–4
).
Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability, loss of efficacy, and increased immunogenicity (
5–7
). Thus, controlling PTMs is a key task in process development to ensure that manufactured biologics have high and consistent quality.
Many chemical modifications such as oxidation, deamidation, glycation, and isomerization can be introduced during multiple manufacturing steps (
8
). However, other modifications are catalyzed by cellular enzymes expressed by production host cells...
HTTPS://STOCK.ADOBE.COM
The acceptance and implementation of single-use systems (SUS) or “disposables” has increased strongly in bioprocess development and biopharmaceutical manufacturing over the past two decades. Typically, suppliers provide SUS presterilized and ready to use. Using SUS eliminates time-consuming and expensive cleaning procedures (which often require corrosive chemicals and a large amounts of water) and removes the need to perform cleaning validation between batches. The application of SUS reduces the risk of product cross-contamination and increases product and patient safety (
1–5
).
Polypropylene (PP) and polyethylene (PE) are common film-contact materials in disposable bags. Polycarbonate (PC) and cycloolefin copolymer (COC) are transparent polymers that can be used as alternatives to glass, and the elastomers ethylene-propylene-diene monomer rubber (EPDM) and methyl-vinyl-silicone rubber (MVQ) are used in seal applications or for pipes (
6
). Most single-use materials are polymers w...
HTTPS://STOCK.ADOBE.COM
Biopharmaceutical manufacturing consists of multiple processes with complex unit operations. Those include mammalian cell culture in upstream operations and downstream chromatography steps for removing impurities from production streams and purifying the therapeutic biological molecule (
1
). Biomanufacturers need enhanced understanding to ensure the process control and manufacture of safe and efficacious drug products. Process understanding also enables opportunities for improving manufacturing efficiency.
Both process understanding and optimization can be facilitated by leveraging large volumes of biotechnology data — typically generated during manufacturing across an entire process — to develop data-driven models. In turn, such models can be used by process scientists and engineers as digital assistants to support their efforts toward understanding processes, monitoring for early fault detection and diagnosis, and identifying areas for efficiency improvements.
Rapid development ...
Demand continues to grow for clinical- and good manufacturing practice (GMP)-grade plasmid DNA (pDNA) amid the proliferation of therapies based on mRNA and viral vectors. At a newly acquired manufacturing facility in San Diego, CA, Wacker Chemie is expanding its capabilities with a scalable platform for pDNA production. In October 2021, Mack Kuo (associate director of bioprocess development at Wacker Biotech US) showcased the site’s capabilities and described how it can provide comprehensive, customizable services for plasmid production.
Kuo’s Presentation
The San Diego site provides customers with nearly 20 years of experience in pDNA manufacturing. Its staff have released over 100 GMP-grade batches for several pDNA products. Those have ranged in size from 3.7 kb to 11.0 kb and have included supercoiled and linearized plasmids cultured in several
Escherichia coli
strains. Activities for those projects have spanned from cell banking through quality control (QC) release of bulk drug substance.
Finished p...
byMack Kuo
The novel coronavirus pandemic has exposed vulnerabilities in biopharmaceutical industry approaches to supply-chain management. Drug developers and manufacturers have tended to presume suppliers’ access to raw materials for bioprocess components. However, global crises disrupt reliable access. Ari Ojinaka (production manager at Astrea Bioseparations) joined BPI in October 2021 to explore strategies for navigating supply-chain uncertainty. He described how his company seeks to optimize manufacturing capacity, enhance communication with its suppliers and customers, and support a quality-driven company culture.
Ojinaka’s Presentation
Traditional supply-chain strategies remain relevant. Biomanufacturers and bioprocess vendors still should qualify at least two suppliers for critical components and use distribution resource planning (DRP) to secure access to different shipping channels. But focusing on supply-chain
reliability
cannot help companies to mitigate near- and long-term disruptions posed by events s...
During process development (PD), single-use (SU) bioreactors can streamline cell-culture workflows and reduce risks for contamination and operator errors. But transferring cultures from laboratory- to benchtop- and pilot-scale vessels requires consideration of fundamental bioreactor principles. In October 2021, Ann D’Ambruoso (manager of product applications and marketing) and Cristina Bernal Martinez (applications support engineer, both at Getinge) reviewed factors for scale-up success and presented a case study involving transfer of cell cultures to Applikon AppliFlex SU stirred-tank (ST) reactors.
The Presentations
Scale-Up Principles:
D’Ambruoso explained that scale-up involves establishing similar geometric and physical variables across culture volumes and reactor sizes. Selecting a robust vessel design can facilitate that work. For instance, AppliFlex ST bioreactors leverage hemispherical bottoms to ensure effective mixing of culture fluids, and three-dimensional printing enables precise production...
In November 2021, Rathangadhara Nammalwar (manager of protein-based therapeutics at Sartorius) delivered a presentation describing the hallmarks of a robust cell line development (CLD) platform. Focusing on the importance of expression constructs, he then explained how collaboration with external partners has enabled Sartorius to optimize its Cellca CLD platform for mammalian-cell production of therapeutic proteins.
Nammalwar’s Presentation
Nammalwar identified key criteria for selecting a CLD platform, noting first that it should leverage a highly productive cell line that yields high-quality proteins. All raw materials should have fully traceable lineages and come from antibiotic-free processes. The period from gene synthesis to production of a research cell bank (RCB) should be reasonably short. Ensuring platform scalability can bolster productivity and help to reduce time, labor, and costs for media and process optimization.
To meet such criteria, Sartorius has enhanced the Cellca CLD platform. It fea...
Despite safety-related setbacks in the early 2000s, adenovirus (Ad) again is gaining traction as a vector for advanced therapies and vaccines. Thus, Ad production is accelerating. In November 2021, Hana Jug (project manager in process development for viral vectors and vaccines at BIA Separations, a Sartorius company) described how her company’s updated platform process for Ad vectors could enhance their purification, maximizing recovery of critical vaccine components. Jug also highlighted BIA’s abilities to supply chromatographic columns consistently and to support customer method development.
Jug’s Presentation
Jug highlighted the advantages of monolith chromatography for Ad purification. Monoliths contain interconnecting channels. Separation is flow independent therein, so eluent is processed quickly without generating turbulence and shear forces that could damage viral vectors. The channels permit no diffusion, and they lack dead-end pores, minimizing the amount of unrecoverable material. Thus, monolit...
byHana Jug
During a December 2021 presentation, Matthew Lotti (senior research associate in analytical development at Ultragenyx) highlighted difficulties with determining adenoassociated virus (AAV) capsid titers during gene therapy production. With support from Gyros Protein Technologies, Lotti spoke about his team’s development of a method for measuring AAV titers using a Gyrolab xPand immunoassay system.
Lotti’s Presentation
Simple analytical methods such as UV spectrophotometry measure capsid titers most effectively after virions have undergone purification. To test samples from earlier process stages, Ultragenyx previously used enzyme-linked immunosorbent assay (ELISA) kits, but such assays are expensive to run and take four to five hours to generate results. ELISAs also feature a narrow dynamic range (8 × 10
6
vp/mL to 5 × 10
8
vp/mL). Because unpurified samples average between 10
12
vp/mL and 10
14
vp/mL, such materials require significant dilution — and that can diminish assay accuracy.
Ultragenyx worke...
A key priority in today’s investment world is corporate adherence to environmental, social, and governance (ESG) requirements. A company’s ESG score serves as a marker of the organization’s values and as a disclosure mechanism for investors to consider. Many companies now consider ESG scores when making strategic choices, and my group has identified a tangible option.
In 2019, the Children’s Tumor Foundation and CureSearch for Children’s Cancer, launched the Bridge initiative (
1
) in partnership with FasterCures, a division of the Milken Institute. Bridge is a neutral information and matchmaking forum for connecting shelved drug assets (SDAs) with partners that have the expertise and capital needed to develop those medicines.
New Life for Shelved Drug Assets
SDAs are promising medicines that have strong phase 1 safety data and pharmaceutical characteristics but have been deprioritized and “shelved” by their sponsor organizations for under five years for non-technical/scientific reasons. Those can include...
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other countries are still highly underserved regarding the access to functional vaccines against the coronavirus disease. Therefore, an optimized production process to achieve the maximal yield of this adenovirus-based vector system is urgently needed to reduce costs per dose and make this vaccine affordable to everyone. Here we describe the development of a highly efficient upstream process to produce adenoviral vectors expressing the spike protein of SARS-CoV2 at high tit...
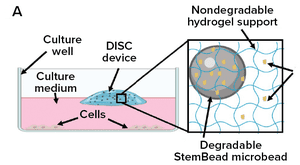
Growth factors, added at the precise time and concentration to in vitro cultures are essential to control cell proliferation and differentiation. When added to a culture vessel, however, the concentrations of these signaling proteins rapidly decline, altering both levels of individual growth factors and the ratio of factors for cell signaling. When growth factors are replenished by exchanging the medium, concentrations peak, resulting in an ebb and flow of growth factor levels resulting in mixed signaling that can lead the cell population to become increasingly heterogenous. Optimizing cell cultures requires control of growth factor levels that drive the desired cell behavior.
Shenandoah Biotechnology and StemCultures solve the challenge of fluctuating growth factor signaling by integrating cell therapy grade, GMP recombinant growth factors with proprietary Defined Inserts for Sustainable Cultures (DISC) devices. DISCs are engineered to provide defined and sustained growth factor levels to cell cultures; ...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.