January-February 2025
When I was supposed to be writing this editorial, I instead was watching the funeral of former US president James Earl Carter of Georgia. The eulogy and speeches by family members and politicians were powerful and moving. The posthumous tribute of Gerald Ford highlighting an unlikely friendship born of rivalry, the inevitable historians’ assessments of a man who sweated the details even to a fault, Joe Biden’s presidential eulogy echoing many previous voices praising Carter’s character and life of service — each one reminded us that after despite recent tribulations, there are ideals worth striving for in a life well lived.
In his efforts toward the nearly complete eradication of guinea worm infection from our species, Jimmy Carter’s legacy bumps up against that of the biopharmaceutical industry. The Carter Center’s work demonstrates the importance of strategies and aspects of treatment that fall outside the realm of medication and vaccination. It serves as a model for addressing other neglected tropical ...
Global advancements in the development of biologics and advanced-therapy medicinal products (ATMPs) are transforming the world of medicine through the approval of novel breakthrough therapies that treat previously uncurable diseases. However, many barriers stand between therapies and widespread access. Some of the most prominent hurdles are high treatment costs, which pose a significant obstacle for patients and other stakeholders, including employers, government-funded healthcare programs, commercial payers, healthcare providers, and drug manufacturers. Shared goals across the industry include improved affordability and accessibility to address health inequities and quality of life (QoL) for patients worldwide.
Across the globe, several initiatives have been established to support biopharmaceutical development and improve patient access. In a 2023 briefing, the US White House Office of Science and Technology Policy presented important goals for the US bioeconomy related health advancements (
1
). Over th...
Regulatory compliance in the pharmaceutical and medical-device sectors is paramount for protecting public health. In the United States, the Food and Drug Administration (FDA) enforces laws and regulations to ensure that products meet stringent safety and efficacy standards. To achieve that goal, the FDA conducts inspection of manufacturing and distribution facilities. Authority for such activities derives from the Federal Food, Drug, and Cosmetic (FD&C) Act, particularly section 704, which grants the FDA broad powers to inspect facilities, records, and products.
The FDA’s guidance document
Circumstances That Constitute Delaying, Denying, Limiting, or Refusing a Drug or Device Inspection
outlines the behaviors and actions that can be deemed noncompliant during such inspections (
1
). The guidance clarifies what constitutes as obstruction of an inspection, helping manufacturers to avoid legal and regulatory repercussions. The document covers a breadth of scenarios, from preannounced inspections to refusal...
From cosmetics to dietary supplements to regenerative medicine, the global market for collagen — and increasingly, vegan and/or plant-based collagen products — has expanded dramatically in recent years (
1
). Some biotechnology entrepreneurs and researchers are responding by leveraging improvements in heterologous protein expression technologies to manufacture recombinant human collagen at commercial scale.
In Canada, PlantForm Corporation is working to meet the growing demand for collagen products by using synthetic biology to “grow” bioidentical human collagen at commercial scales. This company aims to share in the global market for vegan collagen, which is valued at over US$6.4 billion and expected to reach $11.4 billion by 2030 (
2
).
“By using plants as biofactories to produce the target proteins,” says Don Stewart, PlantForm’s president and chief executive officer (CEO), “we can provide a low-cost and highly scalable source of collagen that matches the mechanical properties and functionality of natu...
The biopharmaceutical market in China has been growing rapidly due to increasing healthcare needs, government support, and investments in research and development (R&D). The market size is expected to grow from about US$48 billion in 2020 to $112 billion in 2025, a 135% increase in five years, with a compound annual growth rate (CAGR) of over 18% (
1
). Such growth makes China an increasingly important player in the global biopharmaceutical industry, offering potential market opportunities to both domestic and international companies.
Accordingly, the evolving biopharmaceutical landscape in China has attracted global attention. In March 2024, leading pharmaceutical chief executive officers (CEOs) gathered in Beijing and demonstrated their commitment to expanding operations within the country (
2
). That high-profile assembly highlighted China’s importance as a key market for biopharmaceuticals — driven by its large population, increasing healthcare needs, and progressive regulatory reforms. As China moder...
Lessons in Bioreactor Scale-Up, Part 5: Theoretical and Empirical Correlations for Predicting the Mass-Transfer Coefficient in Stirred-Tank BioreactorsLessons in Bioreactor Scale-Up, Part 5: Theoretical and Empirical Correlations for Predicting the Mass-Transfer Coefficient in Stirred-Tank Bioreactors
A primary difficulty associated with scaling up cell-culture bioreactors involves prediction of physiological conditions in target vessels. As discussed earlier in this series, oxygen availability — characterized by its rate of transfer to the culture liquid (
oxygen transfer rate
, OTR) and its consumption by cells (
oxygen uptake rate
, OUR) — is critical to proper cell function and production of desired proteins. Cellular respiration and production in a large-scale bioreactor depend on maintaining adequate oxygen levels throughout the bioreactor without any gradients.
In a stirred-tank bioreactor (STR), a gas or a mixture of gases often is sparged through the liquid as bubbles. Fractional gas hold-up in the bioreactor is the primary measure of gas–liquid contact efficiency. Hence, gas hold-up (
Φ
) and mean bubble diameter (
d
b
) determine the gas–liquid mass-transfer area. In turn, the most important characteristics affecting mass transfer between gas and liquid phases in an STR are
• gas hold-up (
Φ...
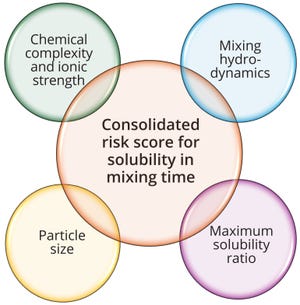
In biopharmaceutical manufacturing, validation of solution-mixing processes plays a vital role in ensuring drug-product quality and regulatory compliance. Because biologics are complex, multicomponent solutions, their successful production hinges on consistently homogeneous mixing. Variations in that process can diminish product efficacy, stability, and safety. Given regulatory agencies’ increasing focus on process consistency, mixing times must be validated comprehensively yet efficiently.
The frequently applied matrix and bracketing methods enable optimization of mixing validation across different solution formulations. Both approaches aim to identify and validate worst-case scenarios, streamlining the validation process while meeting regulatory requirements. However, such studies must be supported by a robust, quantitative risk-assessment framework that includes key factors influencing mixing effectiveness.
We introduce such a framework, evaluating mixing performance as a function of hydrodynamic condi...
A proliferation of biotechnology start-ups and increased commercialization-capacity needs for biopharmaceutical companies have led to a paradigm shift from traditional in-house to external product-development and commercialization with the help of contract development manufacturing organizations (CDMOs). Such partnerships work on the underlying assumption that a process will be transferred successfully between companies and/or production sites, a process commonly referred to as
technology transfer
, which hinges on manufacturing requirements to meet clinical and commercial needs.
The Q10 guidance document from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides a clear and comprehensive description of technology transfer as follows:
Technology-transfer processes require active management from small-scale process development through intermediary pilot scale and subsequent transfers to clinical and/or commercial manufacturing plants, either...
The biopharmaceutical industry increasingly is embracing digital technologies, automation, and data analytics to enhance quality and expedite drug development and manufacturing. Such advances allow for precise control and optimization of complex processes through solutions ranging from artificial intelligence (AI)–driven predictive models to real-time data analysis. With improved accuracy in forecasting production outcomes, advanced digital technologies can reduce errors and boost overall efficiency.
Samsung Biologics has integrated digital-twin (DT) technology as part of its commitment to fostering innovation, enhancing transparency with clients, and increasing responsiveness during manufacturing. A DT-based approach not only boosts operational efficiency, but also increases customer satisfaction through improved process control and rapid decision-making capabilities.
Process Simulation and Optimization:
DT technology creates a virtual model of physical processes, enabling scientists to explore “what-if...
Manufacturing adenoassociated viruses (AAVs) for gene therapies is a complex process involving a series of critical steps to ensure that final products meet stringent quality and safety requirements. AAVs are used widely for in vivo gene-therapy applications, with significant contributions from industry leaders such as Genentech (a member of the Roche group). Such companies have spearheaded innovations in advanced-therapy medicinal products (ATMPs), working with partners including Sangamo and BioNTech to advance the field. In an October 2024 Ask the Expert webinar, Roland Pach (global analytics expert at Roche CustomBiotech) discussed potential critical quality attributes (CQAs) of AAV products and the analytical techniques used to ensure that those attributes are achieved during AAV manufacturing.
AAVs require coinfection with other adenoviruses for replication. AAVs have genetic payload capacities of ≈4.7 kB, limiting the amount of transgenic material that can be inserted. The wild-type AAV genome conta...
The biopharmaceutical industry is undergoing rapid growth, driven by increasing demand for biologics such as monoclonal antibodies (mAbs), other recombinant proteins, and vaccines. However, that growth presents challenges in scaling production processes while maintaining product quality and efficiency. Increasing viable cell densities (VCDs) in culture without compromising final product quality is especially crucial for the success of upstream development in biomanufacturing. In a September 2024 Ask the Expert webinar, Hakyung Kim and Dongyoung Park (senior scientists in upstream process development at Samsung Biologics) presented some of the latest advancements in cell-culture technology and their company’s S-Tensify platform for cell-culture process efficiency.
Limitations of Fed-Batch Cultures:
Biotherapeutic production requires manufacturing processes that maintain sufficient productivity and high product quality. However, achieving the necessary balance between scalability, productivity, and quality...
Transitioning from batch to continuous manufacturing requires scientists to overcome a number of obstacles. In continuous manufacturing, real-time process control is critical, requiring advanced-monitoring systems and automation to maintain consistency and product quality. Such systems can be difficult to implement, and they require frequent adjustments. Integrating upstream and downstream operations also must be seamless, requiring synchronization across fermentation and purification. Biologics are sensitive to environmental fluctuations, complicating product-quality management over long production periods.
Regulatory frameworks traditionally are built around batch processes. Continuous systems necessitate new approaches for validation, real-time release testing (RTRT), and compliance. Designing new facilities or retrofitting old ones makes for an expensive transition, especially when considering the complexity of ensuring supply-chain consistency. Handling failures is particularly challenging in continu...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.