The pandemic forced many companies in many industries around the world to rethink how to get work done with much less in-person interaction than ever before. For the few of us who already worked from home at least part time, it wasn’t quite business as usual — but only because the circumstances brought technological and procedural improvements in how we work remotely. For most of our colleagues, however, it was a bit like getting tossed into the deep end of the pool with only a pair of inflatable armbands to help keep them afloat. I suspect that some of you can relate.
Many companies (our own Informa among them) have reassessed the situation, however, as vaccines have become more widely available over the past year or so. Wiser leaders have sought not a return to the old normal, but rather establishment of a new one that could be more sustainable over the long term. In many cases the solution has included what our company calls “balanced working,” with colleagues spending some time at home and some time t...
STOCK.ADOBE.COM
I was both excited and curious the day I started a new job in the quality affairs department of a pharmaceutical company. As usual, I attended onboarding meetings and received a training plan that contained a list of standard operating procedures (SOPs). So far, so good.
But once I looked into the company’s training documents, my excitement turned to surprise. The SOP training included more than 150 good practice (GxP) SOPs, several of them longer than 50 pages and full of undefined abbreviations and references to unincluded documents. Some SOPs described legislation but not how it translated to defined company processes. Other SOPs were job descriptions for key personnel; interesting to read, but unrelated to my area of work.
Although my experience may not have been typical, as a GxP auditor and consultant, I realized quickly that many companies have room to improve their implementation of SOP systems. That prompted me to consider deeply how to change the popular perception of SOPs from b...
HTTPS://STOCK.ADOBE.COM
The SARS-CoV-2 pandemic created unprecedented stresses on bioprocessing and healthcare supply chains as suppliers struggled to find materials to meet demand for COVID-19 vaccines and therapeutics. BioPlan Associates, with BioPhorum and its members, recently researched the biomanufacturing industry’s response to the pandemic and prepared a white paper,
Impact of COVID-19 on the Bioprocessing Supply Chain
(
1
). Before COVID-19, the bioprocess supply industry had been growing consistently at 12–14% (nearly doubling revenue every five years) since 1990. During COVID, growth in supplier revenue increased dramatically, averaging 27% year-on-year growth (
2
). However, post-COVID, the supplier landscape has changed, possibly permanently.
Supplier Crisis on the Horizon?
We initiated our research because some industry suppliers had expressed concerns about recent order reductions and cancellations for consumables as the COVID pandemic began to abate. Debates emerged about whether biologi...
Production of SUS equipment in a cleanroom.
Particulates are mobile, undissolved particles other than gas bubbles that are unintentionally present in an injectable drug product. Patient safety can be compromised by the amounts and types of particulates present in a drug product (
1
). They differ in nature (e.g., metal, glass, dust, fiber, rubber, polymer, mold, and degradant precipitates) and can be divided into three categories: intrinsic, inherent, and extrinsic particulates.
Intrinsic (native) particles
are derived from operation of a manufacturing process or its equipment, a product formulation, or a container system.
Inherent (formulation) particles
are innate product characteristics (e.g., adjuvants in vaccines and lipid nanoparticles (LNPs)) used as drug delivery vehicles).
Extrinsic (foreign) particles
originate from a manufacturing environment and are foreign to a manufacturing process.
Regulatory requirements for final drug products and biopharmaceutical manufacturing processes are clear. Th...
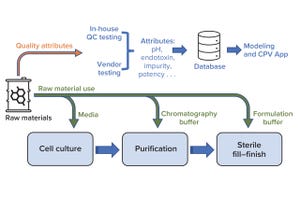
Biologics manufacturing entails multiple complex unit operations across three key process areas: cell culture, purification, and sterile fill–finish (Figure 1). Numerous raw materials are used to formulate reagents that are vital to those processes. For example, bioreactors require cell-culture media, and buffer solutions are used during both drug-substance filtration and drug-product final formulation. Changes in raw-material properties can introduce variation in the performance of intermediate processes and in product quality attributes. Therefore, raw-material properties must be monitored to help ensure consistently high product quality.
Figure 1:
Overview of raw-material use in key biomanufacturing processes (cell culture, purification, and sterile fill–finish) and a high-level workflow for raw-material data use during quality monitoring; CPV = continuous process verification, QC = quality control.
Continued process verification
(CPV) is a formal framework for ensuring that a biomanufacturing proces...
Before June 2015, pharmaceutical manufacturing of certain types of drugs required dedicated facilities. The European good manufacturing practice (GMP) guidelines state that to minimize the risk of cross-contamination, “dedicated and self-contained facilities must be available for the production of particular medicinal products, such as highly sensitizing materials (e.g., penicillin) or biological preparations (e.g., from live microorganisms). The production of certain additional products, such as certain antibiotics, certain hormones, certain cytotoxics, certain highly active drugs, and nonmedicinal products should not be conducted in the same facilities” (
1
).
In multiproduct manufacturing facilities that do not handle those “certain” types of drugs — which were never defined — acceptance-limit calculations for cleaning procedures followed three simple rules first articulated by Fourman and Mullen in 1993 (
2
):
• No more than 0.001 dose of any product will appear in the maximum daily dose of another pr...
Minimizing a facility footprint while maximizing manufacturing capacity is essential to staying agile, productive, and cost-effective — all of which are key elements to competing in a dynamic business landscape.
To achieve such efficiency at commercial scale, bioprocessing facility design should be tailored to each organization’s specific needs. During scale-up, tailor-designed facility planning is critical to streamlined manufacturing of high-quality products. The size and layout of a space can otherwise become a limiting factor for long-term productivity, revenue, profit, and regulatory approval. Therefore, early consideration of operations is essential.
A number of factors will shape planning decisions: Modalities, operations, and budgets can encourage biomanufacturers to incorporate single-use (SU) processing and process-intensification strategies to meet target yields, costs, and timelines. Such next-generation manufacturing approaches will introduce specific layout needs to an overall design strateg...
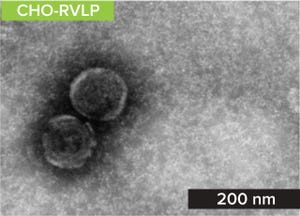
Early evidence of budding C-type particles from Chinese hamster ovary (CHO) cells was documented in the 1970s and 1980s (
1, 2
). In the early 1990s, scientists from Genentech began to characterize these “retrovirus-like particles” (RVLPs) from CHO cells (
3
). Although proven to be noninfectious (
1, 2
), the endogenous particles caused regulators to require a demonstration of retrovirus clearance before clinical trials or market approval authorization (
4
). To accomplish that, the biopharmaceutical industry adopted the use of xenotropic murine leukemia virus (XMuLV) as a model retrovirus for contract research organization (CRO)–led spiking studies. In the meantime, scientists continued to characterize CHO-endogenous RVLPs, sequencing the proviral genome (
5
) and using that information to develop highly sensitive detection assays based on real-time quantitative polymerase chain reaction (RT-qPCR) (
6
). Not long after that, process development scientists began leveraging that knowledge to conduct CHO-e...
Photo 1
: Single Use Support’s universally applicable RoSS.pFTU XL plate-based freezing platform.
Valuable bulk biopharmaceutical intermediates, such as bacterial or yeast cells for microbial fermentation or materials produced during a biomanufacturing process, often are transported to and from different manufacturing sites. During that transfer, maintaining product quality while minimizing degradation in desired properties is of utmost importance. It requires carefully controlled and reliable cold-chain logistics, which in turn rely on the equally carefully controlled process steps of freezing and thawing.
Spray drying is a widely used method for preserving microbial cells. However, some industry insiders point out that product losses often exceed 30% when such a process is applied to monoclonal antibodies (MAbs) or vaccines. An alternative is to freeze the liquids, but until now, freezing large volumes generally has been avoided because of technological limitations. Established equipment (e.g., static a...
Although gene therapies are associated with treating rare diseases, candidate therapies for prevalent-disease indications have overtaken rare-disease treatments in clinical trials, including phase 3 studies. That indicates a change in momentum for new gene therapies that will bring innovative drug products to large patient populations. In an April 2023 webinar, Dovile Gruzdyte, global product manager for cell-line development at Cytiva, discussed how evolving needs in gene-therapy bioprocessing are driving advancements in cell-line development.
Gruzdyte’s Presentation
Gene therapies for prevalent indications are approaching commercialization, which will exacerbate already high demand for production materials. Gruzdyte said that gene-therapy manufacturing processes currently lag behind scientific progress, with many companies still relying on steps and methods that were designed for monoclonal antibody (MAb) production. To match the impending demand for new therapies, developers will need to scale up their...
Although advanced digital solutions have become essential to the success of biopharmaceutical companies in recent years, a December 2022 survey from healthcare and life-sciences consultancy Sage Growth Partners (Sage) found that existing options often fail to meet key needs. The survey identified the plans, preferences, and needs of biopharmaceutical digital-solution buyers based on the answers of 116 respondents from precommercial mid- and large-size companies across the United States. The survey evaluated six biopharmaceutical-solution areas: analytics, real-world evidence (RWE), enterprise, clinical development, patient engagement, and digital therapeutics. Sage found market fragmentation in multiple areas and discovered that many solution providers struggle with low-unaided brand awareness. However, a surprising number of respondents indicated a willingness to work with small and emerging solution providers to improve their capabilities.
Despite an industrywide trend toward digitization, data integrat...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.