https://www.lifetouch.com
No matter what topic you delve into these days in the biopharmaceutical industry — e.g., process development, market projections, equipment scaling, quality control, regulatory harmonization — you’ll find a tangled web. Development of vaccines and therapeutics for prevention and treatment of COVID-19 continues to play out against the backdrop of unrelated products in development and others already on the market as short-term manufacturing needs and diversions of contract capacity shift the industry’s focus. This issue provides only a snapshot of how facilities are housing such efforts and how flexible designs can support short- and long-term planning.
The industry was more ready to meet the current challenges than it would have been at any other point in its history. A number of innovations have enabled companies to work quickly while managing risks inherent to such speeds. Vital technologies have become available at just the right time. As the Corning Life Sciences director of s...
Pharmaceutical facility cleanrooms are designed to reduce and control particle contamination and to minimize the ingress and retention of microorganisms. Such risks typically are easy to control in well-designed, modern facilities. But risk mitigation is more difficult in older facilities.
The former Idec Pharmaceuticals site on Torreyana Road in San Diego was closed in 2011 after the company merged with Biogen. Four years later, it became home to the Biocom industry trade group’s California headquarters. Photos circa 2004, courtesy of Biogen (
https://www.biogen.com
)
There is no exact definition of what constitutes an aging facility (or what are sometimes euphemistically called
legacy facilities
). For example, a facility established 100 years ago to manufacture a simple tablet can continue to operate perfectly well with careful upkeep and an eye on developing regulations. By contrast, a biomanufacturing site that was established 10 years ago can become out of date if it doesn’t receive necessary proce...
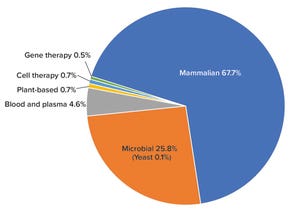
Figure 1: Percentage of total biomanufacturing capacity by platform or system
Since 2018, global bioprocessing capacity has grown from 16.5 million liters (
1
) to 17.4 million liters. Although output has continued to expand at around 12% overall, that rate represents a significant slowing in capacity growth as the industry moves toward greater productivity and efficiency. Trends that we have tracked in the BioPlan Associates annual report of biopharmaceutical manufacturing capacity and production (
2
) for over 17 years correlate with that finding. Titers are increasing; single-use technologies have reduced the need for large stainless-steel capacity; and the decline in approvals for large-volume, blockbuster biologics has diminished the need for super-size, “six-pack,” and multiple 10,000-L facilities worldwide. Optimizing capacity use will require planning for flexibility and peak-demand strategies. Further, the near-universal push toward greater productivity has led to commissioning of more facilities...
Gilbane completed this facility for Alnylam Pharmaceuticals, Inc. (Norton, MA) in January 2020. (
https://www.gilbaneco.com
)
In the early 2000s, the trade press was abuzz about an imminent “capacity crunch” in mammalian cell culture. Dire predictions of shortages were based on biopharmaceutical successes to that point, on bursting development pipelines, and on the lengthy timelines and high costs of assembling tens of thousands of liters of stainless-steel bioreactors and supporting infrastructure.
Those predictions failed to anticipate several positive developments that would render doom-and-gloom scenarios moot. Notably, yearly improvements in protein titers for MAb processes already were raising volumetric productivity from tens of milligrams per liter to as high as 10 g/L for monoclonal antibody (MAb) manufacture in Chinese hamster ovary (CHO) cells (
1
). Simultaneously, companies were beginning to apply single-use technology that previously had been limited to buffer preparation and hold tanks to m...
https://www.gconbio.com
Biomanufacturers seeking the best approach to rapid implementation of flexible manufacturing capacity take into account the benefits presented by different modular construction options. We analyzed different approaches to building manufacturing capacity and assessed the economic benefits of each approach. Our evaluation was based on biopharmaceutical products for which there is an immediate unmet need, such as treatments or vaccinations for COVID-19. Such products also might entail a sudden increase in demand (e.g., expansion of a product indication or sales ramp up faster than expected). The timing required for increasing capacity is influenced by many factors, including those related to business, technologies, and regulatory compliance. Modeling techniques can be used to mitigate investment risks associated with manufacturing facilities.
Capacity Challenges
A rapid buildup of manufacturing capacity is required when there is a huge unmet need for a drug product. Although some opti...
Many veterans of the biologics industry presume that emerging therapeutics such as cell and gene therapies (CGTs) require production facilities that differ substantially from those for monoclonal antibodies (MAbs) and other conventional biologics. But experience with designing CGT facilities bears out that far more synergies than differences exist across facilities for conventional and advanced therapies. Herein, I call attention to some of those shared design concerns and demystify facilities and engineering requirements for CGTs.
Observing the Synergies
Many processes have evolved from adherent-based cell lines and processes, and although some commercial processes still use those platforms, most now operate as suspension platforms with scalability and operational advantages. Gradually, viral-vector gene therapy processes have transformed so that they can produce at scales on par with small-scale MAb facilities. Of course, the clearest difference between production of gene therapies and MAbs is that the ...
It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping to ensure timely production, but it also would help patients by increasing their access to the therapies they need.
Industry groups such as BioPhorum, ASTM International, and some individual suppliers have made attempts to influence widespread adoption of single-use standard designs, but most of those programs have been unsuccessful in achieving that goal. The situation can be attributed to a number of barriers preventing substanti...
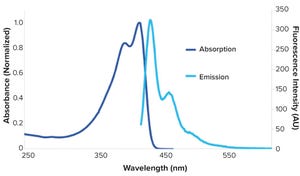
Figure 1: Absorption and emission spectra
(λ
exc
= 405 nm) of SuperNova v428 in phosphate-buffered saline (
2
)
Since the emergence of SARS-CoV-2, scientists have discovered more about the role of our immune systems and cytokine-associated processes responsible for systemic immune reactions that are typical in patients with COVID-19 (
1
). Flow cytometry is used to understand those processes at a single-cell level. It is the standard method used in immunology to characterize multiple phenotypic and functional parameters of single cells, including cytokine analysis.
Despite advances in flow cytometry instrumentation, reagents, and analytical software, several challenges remain. Conjugated antibodies with improved brightness, stability, and specificity are needed. They also need to have narrow excitation and emission spectra so that different fluorochromes can be used in combination. That would enable scientists to analyze multiple parameters from one sample simultaneously.
Low sensitivity also is a proble...
Rapid growth and changing market dynamics in biologics and vaccine sectors have prompted biopharmaceutical companies and contract development and manufacturing organizations (CDMOs) to build new manufacturing facilities at unprecedented speeds. This often means developing single-use (SU) or hybrid bioprocessing facilities with reusable legacy equipment for production of monoclonal antibodies (MAbs), antibody–drug conjugates (ADCs), and viral-based vectors.
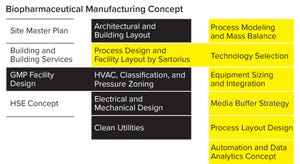
Figure 1: Work packages of a biopharmaceutical manufacturing concept highlighting process design and facility layout (HSE = health, safety, and environment)
For many companies, facility design begins by engaging an engineering consultancy to develop a layout that maximizes space use and minimizes equipment, fittings, and operating costs. SU technology suppliers are seldom consulted during the planning stage. Users assume that fitting SU equipment will not present a key facility design challenge, so they give more weight to other factors early on. Howeve...
Although formulation and drug-product activities are critical to developing gene therapies, much remains to be learned about their degradation mechanisms, and firm criteria still need to be established for buffer and excipient selection.
Sarathi Vijay Boddapati
(associate director of formulation and drug development at Catalent Cell and Gene Therapy) joined BPI on 25 March 2021 to present what his company continues to learn about formulating gene therapies by adapting methods used for other biologics.
Boddapati’s Presentation
Adenoassociated virus (AAV) remains the most common vector for gene therapies. As with biologics, formulation and drug-product activities for AAV products fall into three categories. The most critical work involves
formulation development and product characterization
, which help to investigate degradation propensities and identify mitigation strategies.
Drug product (DP)-related activities
determine how a product will be stored, handled, and administered. Freeze–thaw and stabil...
The push to expedite COVID-19 vaccines over the past year has led the biopharmaceutical industry to try novel strategies to accelerate product development and manufacturing. To compress discovery, development, and manufacturing into several months rather than a typical multiple-year effort has meant reexamining nearly every aspect of the process — including facilities required to house the work.
Successful COVID-19 vaccine development undoubtedly will change biomanufacturing strategy forever. Previously, current good manufacturing practice (CGMP) manufacturing options were twofold:
Now a growing number of early stage companies are considering a novel hybrid approach to leasing cleanroom space, with related infrastructure and expertise provided as a service. This model eliminates facility build-out lead times, shifts the burden of facility maintenance, and prevents the need to share intellectual property (IP) with third parties. Flexibility and control afforded by a hybrid strategy give developers the auto...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.