This issue goes to production as we editors board our flights from Eugene, OR, to the east coast for Biotech Week Boston. As you can imagine, it’s our biggest show of the year — and it will be my first time back since the pandemic disrupted everything three years ago. I have missed greatly our yearly get-together with far-flung staff members from Europe and across the United States. It’s almost like a family reunion with all the associated logistical complications and not-enough-time laments. We’re very grateful for Informa’s Streamly platform these days — as are most conference-goers, I’m sure — because it’s hard to catch every interesting talk between meetings and parties and casual encounters with favorite authors and advisors in the corridors and exhibit hall. If you see us anywhere, don’t hesitate to stop and chat because that’s one of our favorite parts of business travel.
That’s partly because we’re always looking ahead to the next issue, next featured report, next eBook, and so on. Already our 202...
STOCK.ADOBE.COM
If you are an ambitious life-science professional seeking to create the next big innovation, starting your own company can enable you to share your ideas with the rest of the world. Opportunities abound within the industry, as shown by the frequent innovative breakthroughs that drive our professional lives. However, although you may be an expert within your industry, it takes careful planning and specialized knowledge of the business world to channel your expertise into a successful new company. Here, you’ll learn key elements to launching a biotechnology start-up so that you can transition from industry professional to entrepreneur.
I
deation and Conceptualization
Strong ideation is the first step to building a new business. The best business ideas solve problems, even if those problems are unrecognized by the people who have them. “Ideas for innovative biomanufacturing technologies are inspired by observing and pinpointing problems that have yet to be solved. Although you can be successf...
HTTPS://STOCK.ADOBE.COM
In our current financial climate, biotechnology companies are facing significant funding difficulties that necessitate careful decision-making when it comes to outsourcing biomanufacturing processes and balancing budgets. Reliable, high-quality bioproduction is paramount to success. Considering the complex nature of biomanufacturing and the intricate requirements involved, biotechnology companies should choose contract manufacturing organizations (CMOs) that operate within current financial constraints and that possess the expertise, regulatory compliance, and technological capabilities necessary to ensure seamless technology transfer and high product quality. Therefore, CMO selection is important to preventing manufacturing delays, supply-chain disruptions, and setbacks with clinical programs, development timelines, and critical milestones. By exploring the complex landscape of CMO selection, process sponsors can make strategic decisions and develop effective partnerships, helping...
ASTRAZENECA (
HTTPS://WWW.ASTRAZENECA.COM
)
Biopharmaceutical manufacturing companies create life-saving medications and treatments that are crucial to global healthcare. It is an industry in which minor production issues can lead to dire consequences, including compromised product quality and regulatory noncompliance, not to mention danger to patients. Thus, proactive equipment maintenance is indispensable. Below, I investigate why proactive maintenance is vital to pharmaceutical manufacturing operations and highlight its role in securing regulatory compliance, reducing facility downtime, improving product quality, and managing risks.
Understanding Proactive Maintenance
Throughout its many complex processes, biomanufacturing demands strict quality adherence. To meet the highest standards consistently, the biopharmaceutical industry is turning to a strategy of proactive maintenance.
Definition and Explanation:
Proactive maintenance
is a way to anticipate and resolve potential problems before they crop ...
HTTPS://WWW.ISTOCKPHOTO.COM
In multiproduct biopharmaceutical manufacturing facilities, cross-contamination with pharmacologically active proteins must be controlled in a good manufacturing practice (GMP) environment (
1, 2
). Although guidance on control strategies exists for solvents and small-molecule pharmaceutical impurities, that is not directly applicable to inactivated (e.g., denatured and/or degraded) therapeutic proteins (TPs) occurring as impurities in a drug substance (DS) and/or drug product (DP). Small-molecule drugs and TPs differ in their molecular structures, pharmacological mechanisms of action, hazards, and potential impurities, so their cross-contamination control strategies also should be considered differently. Unlike small molecules, TPs are known to denature and degrade when exposed to pH extremes and/or heat and thus are expected to become pharmacologically inactive during the cleaning process (
2
).
In cleaning activities, permitted daily exposure levels (PDEs) support the amount...
Packed-bed chromatography is a vital downstream operation for purifying valuable biological products, including monoclonal antibodies (MAbs) and emergent therapeutic modalities such as viral vectors. Conventional chromatography unit operations in bioprocessing use highly porous microspheres packed into cylindrical columns, purifying complex feed streams through characteristics such as size, charge, and hydrophobicity. The porosity of both a packed bed and its constituent beads relates directly to the intended function and optimal performance in terms of both chemical and physical separation (
1
).
High-resolution imaging techniques have developed sufficiently to the point at which they can be used to visualize and characterize complex geometries such as packed columns. These methods help us understand the detailed, internal structure of many different materials (
2
). X-ray computed tomography is an effective method for imaging at nanoscale resolutions in three dimensions (3D) while negating the need to s...
Control of Host Cell Proteins in Monoclonal-Antibody Bioprocessing: Using Proteomic Analysis To Understand Impurity Clearance and Persistence During PurificationControl of Host Cell Proteins in Monoclonal-Antibody Bioprocessing: Using Proteomic Analysis To Understand Impurity Clearance and Persistence During Purification
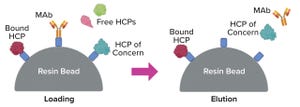
Downstream process development can proceed like a detective novel, starting with evidence of something seriously wrong and rapidly evolving into a “whodunit.” The evidence often comes as precipitate particles in what is supposed to be a stable formulation. The whodunit takes the form of root-cause analysis into the degradation mechanism of a biopharmaceutical product or of a key ingredient in its formulation. And the culprit often turns out to be an enzyme present in such small quantities as to be almost undetectable. The rash of such cases during manufacturing of monoclonal antibodies (MAbs) and other biopharmaceuticals has changed the field of impurity clearance, including associated assays and control strategies.
Issues with impurity clearance stem almost invariably from host cell proteins (HCPs). Along with host-cell DNA (hcDNA), cell debris, lipids, and viruses, HCPs are categorized as
process-related impurities
, distinguishing them from
product-related impurities
such as product aggregates (ofte...
When you publish with BPI, you reach nearly 100,000 global readers working in all phases of biopharmaceutical development and manufacturing. If you have a topic that you want to develop into an article, or if you are seeking a “home” for a manuscript, contact
managing editor Brian Gazaille
(
[email protected]
). He can let you know of our interest and potential publication timelines. We are happy to respond to drafts, but unsolicited manuscripts are welcome.
What We Publish
“Focus On . . . ”
(nontechnical) articles of ~1,500–3,000 words explore regulatory trends, business issues, risk management strategies, industry training, bioethics, and other topics relevant to the biopharmaceutical industry.
Peer-reviewed (technical) articles
usually run ~2,000–5,000 words. These are the “meat” of the magazine, providing specialist-level analyses on biomanufacturing and drug development for a breadth of biotherapeutics. Such articles may be detailed case studies, descriptions of industry “best practice,”...
Trelleborg experts leverage a reinforced polypropylene (PP) composite material to make thin, light chromatography columns, using significantly less material than is needed for traditional options.
Recent years have witnessed biopharmaceutical manufacturers transition swiftly from traditional stainless-steel systems that require harsh sterilization between applications to single-use systems (SUS) that are less expensive, faster to produce, and — perhaps counterintuitively — more compatible with sustainability initiatives (
1
). Now that disposable systems have become industry standard, biopharmaceutical original equipment manufacturers (OEMs) are seeking full-service components partners that can offer further innovations in SUS.
Integrated Solutions
Some suppliers can provide biopharmaceutical OEMs with tailored assembly solutions in addition to finished components. Such work could involve integrating connectors or sensors to extruded tubing assemblies and providing complete system assemblies such as singl...
Global access to medication is a crucial driver in the pharmaceutical industry (
1
). Thus, drug manufacturers are encouraged to lower their production costs while increasing productivity to bring affordable drugs to market quickly.
Process intensification is a natural solution for improving facility output. So far, upstream processes have been the main focus of intensification efforts. Combined with high-performing cell lines, those strategies have created higher titers. However, manufacturers now face bottlenecks in their downstream processes, which must evolve to handle the improvements.
Downstream process intensification is an ideal solution for solving such issues. Process intensification can increase yield, decrease process timelines, reduce cost of goods (CoG), reduce footprint, and increase flexibility without making significant changes to process parameters. The relative importance of these drivers will inform the selection of an intensification approach. As the workhorse of downstream bioprocess...
The global biotechnology industry has undergone a significant period of growth over the past three to four years. The COVID-19 pandemic accelerated the size and importance of an already growing sector, compounding the responsibility that falls on developers and manufacturers to deliver products that are uncontaminated and safe. Ensuring compliance with industry regulations is essential to safety, and professional attire for laboratories and cleanrooms is an integral part of adhering to standards.
Biomanufacturing businesses often handle living cells, cell components, live viruses, and other potentially sensitive or harmful substances. Such work involves adhering to strict safety and cleanliness standards. Because of the potential hazards of bioprocessing, the industry is heavily regulated. Depending on the nature of their work, companies may be subject to rules imposed by the US Department of Agriculture (USDA), Environmental Protection Agency (EPA), Occupational Safety and Health Administration (OSHA), a...
Subscribe to receive our monthly print or digital publication
Join our 70,000+ readers. And yes, it's completely free.